Abstract
Background: A majority of younger pts with AML receive initial therapy with a 7+3 scheme. Escalated doses of daunorubicin (dauno) in combination with standard doses of ara-C may lead to improved outcomes (Fernandez; NEJM 2009). A phase II trial of idarubicin and high-dose ara-C (IA) in combination with the histone deacetylase inhibitor vorinostat (IA+V) resulted in historically high response rates compared to IA or 7+3 (Garcia-Manero; JCO 2011). SWOG 1203 tested whether a high-dose ara-C induction with or without vorinostat could result in improved outcomes for younger AML pts compared to 7+3.
Methods: The primary endpoints were comparison of event-free survival (EFS) of 7+3 vs IA vs IA+V and evaluating the frequency of allogeneic hematopoietic cell transplantation. Secondary endpoints included toxicity, remission rate, relapse-free survival (RFS), and overall survival (OS) according to treatment arm, and by cytogenetic and molecular subgroups. Because of the historical trend of cured pts in S0106, EFS was modeled with an exponential cure model, and it was assumed IA or IA+vorinostat increased the proportion of pts cured from 35% to 45% and increased median EFS among those not cured from 4.7 months to 7.1 months. Main inclusion criteria included a diagnosis of previously untreated non-APL AML by WHO criteria, age 15 to 60 years, and preserved cardiac function but no severe comorbidities. Pts with known CBF rearranged or FLT3 mutant leukemias were eligible if no other alternative clinical trials existed. Treatment was as follows: Induction: 7+3 arm: dauno 90 mg/m2 IV QD x 3 on days 1-3 with ara-C CI 100 mg/m2 QD x 7 days on days 1 to 7. IA arm: ida 12 mg/m2 QD x 3 on days 1 to 3 with 24 hours CI ara-C 1.5 gm/m2 QD for 4 days on days 1 to 4. IA+vorinostat was as IA but with vorinostat 500 mg orally TID for 3 days on days 1 to 3. Consolidation: 7+3 arm: standard high-dose ara-c at 3 gm/m2 over 3 hrs q12 hours x 6 doses for 1 to 4 cycles depending on transplant availability. IA arm: idarubicin 8 mg/m2 IV QD x 2 days on days 1 to 2 with ara-C 0.75 gm/m2 CI for 3 days on days 1 to 3 for 4 cycles. The number of consolidation cycles depended on transplant indication; a secondary aim of the study was to transplant all cytogenetically-determined high risk pts (presented in a different abstract).
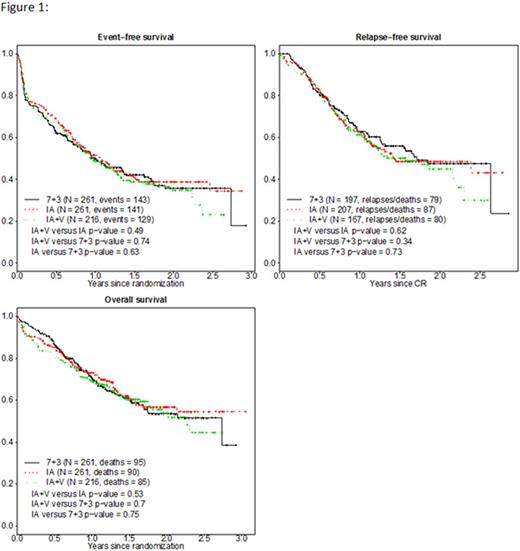
Results: Of 754 pts randomized, 738 were eligible (261 to 7+3, 261 to IA, and 216 to IA+V). Baseline characteristics were well balanced among all three groups. Most (75%) pts were between 40 and 60 years, 51% were male, median (range) WBC and platelets were 10.8 (0.3-800) and 49 (3-3900), respectively; marrow blast percentage was 60% (0%-100%); cytogenetics were favorable in 13% of pts, intermediate in 63%, and high-risk in 22%; FLT-3 was mutated in 21% and unknown in 31%; NPM1 was mutated in 20% and was unknown in 37%. Complete remission (CR) rates were 75% for 7+3, 79% for IA, and 77% for IA+V (p=0.58). Significantly more pts received reinduction with 7+3 (24%) versus 11% with IA and 9% with IA+V (p=0.001). 48% of intermediate and unfavorable risk pts received transplant in CR1, with no significant differences among arms (p=0.44). There were no significant differences in EFS, RFS or OS among all three arms (Figure 1, all p>0.5). By cytogenetic or molecular group, there were no differences in outcome for any standard risk subset (FLT-3, NPM1, or CEBPα or cytogenetic subset), although pts with favorable cytogenetics had significantly better EFS, RFS and OS with 7+3 therapy compared to IA or IA+V (Figure 2, all p≤0.015). 30% of pts received 1 consolidation cycle, 18% 2, 16% 3, and 37% 4 cycles. Grade 5 induction toxicity rates were 4% (7+3), 8% (IA), and 9% (IA+V) by arm (p=0.07), and for toxicities related to therapy, 2%, 7%, 8%, respectively (p=0.01). Grade 4 induction toxicity rates were similar across arms (p=0.38).
Conclusion: Treatment with IA is not more effective than 7+3 in younger pts with AML. Outcomes with IA or IA plus vorinostat are similar. In pts with favorable cytogenetics, outcomes were inferior with IA or IA+V when compared to 7+3, perhaps related to use of lower doses of ara-C during consolidation.
Clinical Trials Registry: NCT #0180233
Support: NIH/NCI grants CA180888, CA180819, CA18020, CA180821, CA180863, CA077202;
CCSRI grant #021039
Othus:Glycomimetics: Consultancy; Celgene: Consultancy. Radich:Novartis: Consultancy, Research Funding; Ariad: Consultancy; BMS: Consultancy. Strickland:Alexion Pharmaceuticals: Consultancy; Astellas Pharma: Research Funding; Baxalta: Consultancy; Cyclacel: Research Funding; Celator: Research Funding; Abbvie: Research Funding; Ambit: Consultancy; Boehringer Ingelheim: Consultancy, Research Funding; CTI Biopharma: Consultancy; Daiichi Sankyo: Consultancy; Sunesis Pharmaceuticals: Consultancy, Research Funding; GlaxoSmithKline: Research Funding; Karyopharm Therapeutica: Research Funding; Sanofi: Research Funding. Savoie:Novartis: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria; Pfizer: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Jazz: Consultancy; Lundbeck: Consultancy. Sekeres:Millenium/Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Stone:Amgen: Consultancy; Karyopharm: Consultancy; Juno Therapeutics: Consultancy; Roche: Consultancy; Pfizer: Consultancy; Agios: Consultancy; Jansen: Consultancy; Xenetic Biosciences: Consultancy; Celator: Consultancy; Merck: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; ONO: Consultancy; Seattle Genetics: Consultancy; Sunesis Pharmaceuticals: Consultancy. Erba:Sunesis: Consultancy; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy, Speakers Bureau; Gylcomimetics: Other: DSMB; Ariad: Consultancy; Astellas: Research Funding; Gylcomimetics: Other: DSMB; Amgen: Consultancy, Research Funding; Pfizer: Consultancy; Gylcomimetics: Other: DSMB; Astellas: Research Funding; Astellas: Research Funding; Amgen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Celgene: Consultancy, Speakers Bureau; Juno: Research Funding; Seattle Genetics: Consultancy, Research Funding; Jannsen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Consultancy, Speakers Bureau; Daiichi Sankyo: Consultancy; Sunesis: Consultancy; Millennium Pharmaceuticals, Inc.: Research Funding; Incyte: Consultancy, DSMB, Speakers Bureau; Agios: Research Funding; Celator: Research Funding; Ariad: Consultancy; Agios: Research Funding; Incyte: Consultancy, DSMB, Speakers Bureau; Jannsen: Consultancy, Research Funding; Juno: Research Funding; Agios: Research Funding; Jannsen: Consultancy, Research Funding; Novartis: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy, DSMB, Speakers Bureau; Juno: Research Funding; Pfizer: Consultancy; Millennium Pharmaceuticals, Inc.: Research Funding; Celator: Research Funding; Daiichi Sankyo: Consultancy; Agios: Research Funding; Celator: Research Funding; Daiichi Sankyo: Consultancy; Millennium Pharmaceuticals, Inc.: Research Funding; Pfizer: Consultancy; Celator: Research Funding; Jannsen: Consultancy, Research Funding; Sunesis: Consultancy; Seattle Genetics: Consultancy, Research Funding; Astellas: Research Funding; Ariad: Consultancy; Ariad: Consultancy; Millennium Pharmaceuticals, Inc.: Research Funding; Seattle Genetics: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Sunesis: Consultancy; Pfizer: Consultancy; Juno: Research Funding; Celgene: Consultancy, Speakers Bureau; Gylcomimetics: Other: DSMB.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal