Abstract
Introduction:Anti-CD19 chimeric antigen receptor T cells (CART19) generate unprecedented complete response rates of up to 90% in relapsing/refractory acute lymphoblastic leukemia. Associated with this therapy is a multi-symptom toxicity known as cytokine release syndrome (CRS). Clinically CRS resembles macrophage activation syndrome (MAS), with a pattern of cytokines in serum coupled with other biomarkers (such as ferritin) and physical findings (fever, sudden organomegaly, confusion). Clinical interest has focused on interleukin-6 (IL-6), as blocking this pathway with tocilizumab (an IL-6R antagonist) has relieved the most life-threatening aspects of CRS in patients. Nothing is known, however, about the mechanism behind the triggering of CAR associated CRS/MAS nor the cellular sources of the toxic cytokines. This is a critical lack of knowledge, as each CAR product may result in a different CRS resulting in different clinical outcomes and management strategies. We sought to identify the cellular source of IL-6 and other MAS cytokines specifically during a 41BB CAR mediated CRS, and cross validate with investigations into patient peripheral blood PBMCs during CRS.
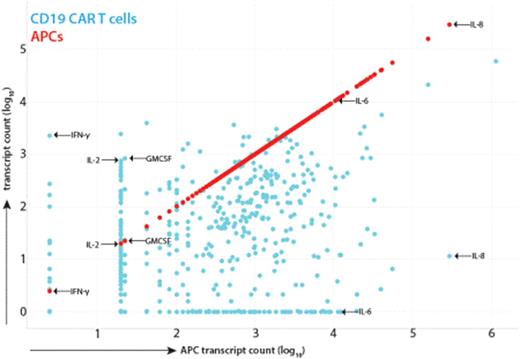
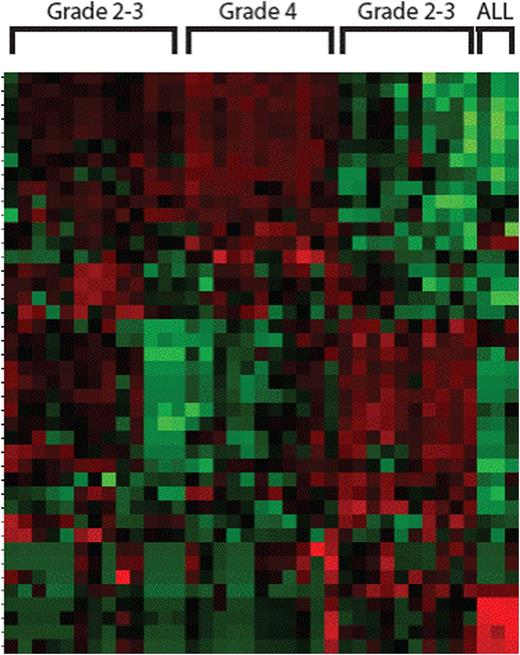
Results: Using a xenograft model of a primary patient leukemia and CART19 from a patient that experienced Grade 4 CRS, we measured cytokine production in the serum of animals 3 and 7 days post CART19. While we could detect GMCSF, IL-2 and IFNg easily, IL-6 was not detected and the animals did not appear ill during this phase despite disease response. Given the clinical similarities of CRS to MAS, we performed co-culture of CART19 T cells, Nalm-6 leukemia and cells derived in vitro from peripheral blood monocytes (which were autologous to the CAR T cells) including immature dendritic cells (iDC), mature dendritic cells (mDC) and macrophages. Similar to the in vivo results, coculture of CART19 and Nalm-6 produced high levels of GMCSF, IFNg, IL-2 and IL-10 but no detectable IL-6 or IL-8. Only in the presence of the monocyte lineage antigen presenting cells (APCs) did we observe IL-6 and IL-8 release (more than 100 fold increase over controls). Transwell in vitro experiments separating CART19/Nalm-6 from the APCs showed the same pattern, indicating the CART19 mediated killing of target cells induces the IL-6 release from APCs in a contact independent manner. Nanostring RNA analysis of separated cell populations indicated that IL-6 and IL-8 are exclusively produced by APCs, not CART19 or Nalm-6 (Figure 1). Both CD107a degranulation and the total Nanostring RNA profile of CART19 was not different in the presence or absence of APCs, indicating that an MAS-like CRS is likely not part of CART19 efficacy. Finally, we analyzed the peripheral blood mononuclear cells of 18 patients receiving CART19 for pediatric ALL by Nanostring. Patients with Grade 4 CRS and only circulating T cells showed no IL-6 or IL-8 RNA, confirming in vivo that CART19 cells are not the cellular source of IL-6 during CRS. Unsupervised clustering of the Nanostring profiles also revealed four distinct gene signatures: one for patients with only circulating leukemic blasts, two for Grade 2-3 CRS that clustered separately and one for Grade 4 CRS (Figure 2).
Conclusions: Here we demonstrate that IL-6 as part of CRS is produced by APCs and not T cells in response to CART19 mediated killing of leukemia, and that CART19 cells do not seem affected by the presence of CRS cytokines either in transcriptional profile or killing potential. This data provides the rationale for blocking this toxic cytokine before symptoms appear without changing CART19 efficacy, in addition to supporting a rapid Nanostring based profile to identify prospectively the patients at risk for Grade 4 CRS.
Scatterplot of RNA transcript levels from CAR T cells (blue) in the act of killing leukemia cells versus the transcript levels from APCs separated by transwell insert. There are clear distinctions on the cellular source of key cytokines in CRS, including IFNg, GMCSF and IL2 from CAR T cells and IL6 and IL8 from APCs.
Scatterplot of RNA transcript levels from CAR T cells (blue) in the act of killing leukemia cells versus the transcript levels from APCs separated by transwell insert. There are clear distinctions on the cellular source of key cytokines in CRS, including IFNg, GMCSF and IL2 from CAR T cells and IL6 and IL8 from APCs.
Unsupervised clustering shows four groups of CRS clusters based on T cell, monocyte and B cell genes. Each column is a patient sample, each row a single gene. Grade 4 is dominated by T cell genes only.
Unsupervised clustering shows four groups of CRS clusters based on T cell, monocyte and B cell genes. Each column is a patient sample, each row a single gene. Grade 4 is dominated by T cell genes only.
Barrett:Novartis: Research Funding. Grupp:Novartis: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal