Abstract
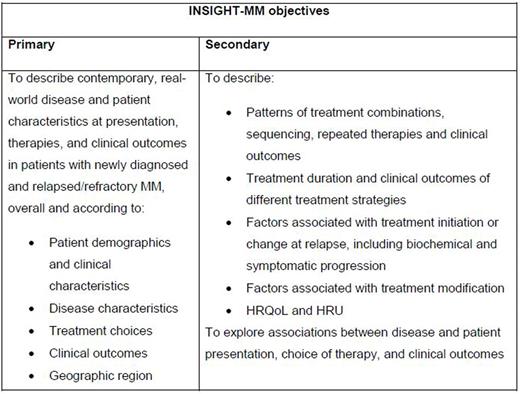
Advances in novel agents and treatment combinations have improved prognosis and increased disease-free and overall survival for patients (pts) with multiple myeloma (MM). However, currently available data on disease presentation, treatment patterns, and outcomes for real-world MM pts at the global level are limited. This is due to several factors, including the overrepresentation of medically fit pts in clinical trials making generalization of outcomes challenging, the large number of treatment combinations, and varying global access/practice patterns. INSIGHT-MM (NCT02761187) is a global, prospective, non-interventional, observational study which aims to further understand disease and pt characteristics at presentation, treatment and clinical outcomes of real-world MM pts, as well as the association of treatment with tolerability, effectiveness, health-related quality of life (HRQoL), and healthcare resource utilization (HRU), on both a country-specific and global basis. As this is an observational study, no formal hypothesis will be tested. The INSIGHT-MM objectives are summarized in the Table.
At least 5000 pts aged ≥18 yrs with newly diagnosed or relapsed/refractory MM will be enrolled over a 3-yr period and followed prospectively for ≥5 yrs, until death or end of study, whichever comes first. Pts not available for data collection for >9 mos will have follow-up for survival. No study drug or medications will be provided; no modification of standard of care pt management will be assigned per protocol. Choice of therapy for all pts will be decided by the treating healthcare provider independent of study participation.
Baseline pt and MM-specific characteristics, diagnosis, comorbidities, and prior therapies will be recorded based on review of hospital/clinic records. MM management, disease status and safety data will be obtained as part of routine office visits and recorded quarterly by each site in electronic case report forms. Quarterly assessment of MM management will be done based on prior and current treatment and recorded reason for treatment changes. Effectiveness of therapy will be assessed based on response, progression status, time to next therapy, vital status, and date and cause of death. Treatment tolerability will be assessed based on serious and non-serious adverse events leading to treatment discontinuation or dose modification. Incidence of second primary malignancies will be recorded.
HRQoL, a specific type of patient reported outcome (PRO), will be collected at study entry and at predefined intervals following initiation of therapy using a secure electronic data collection system. HRQoL will be collected using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and the MM module (EORTC QLQ-MY20). To capture pt satisfaction with MM-directed therapy, including the dimension of convenience, the 9-item Treatment Satisfaction Questionnaire for Medication will be used. The 5-dimension, 5-level EuroQol (EQ-5D-5L) PRO instrument will capture self-reported preference-based measures of health status suitable for calculating quality-adjusted life year (QALY) data to inform health economic evaluations. Frailty will be assessed using the Charlson Comorbidity Index, the Katz Index of Independence in Activities of Daily Living, and the Lawton Instrumental Activities of Daily Living. HRU will be evaluated using inpatient and intensive care unit admissions, length of stay, outpatient clinic visits, and emergency room visits.
Data for all participating pts will be extracted by healthcare professionals at the site level and entered into a central database; descriptive statistical analyses will be done to address the study objectives. Interim analyses are planned after 1000 and 5000 pts have been enrolled and a final analysis will be conducted within 1 year after the last pt entered has completed ≥5 yrs follow-up. Data will be analyzed biannually to address emerging clinical questions identified by investigators to increase understanding of real-world treatment patterns. INSIGHT-MM aims to promote better understanding of contemporary demographics, patterns of care, and outcomes for real-world MM pts to inform treatment practice, supportive care, and pt outcomes. The study is currently ongoing and recruiting pts; further details regarding study rationale and protocol will be provided.
Davies:Takeda: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Palumbo:Janssen Cilag: Honoraria; Takeda: Employment, Honoraria. Zonder:Bristol Myers Squibb: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Prothena: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Pharmacyclics: Other: DSMC membership. Girnius:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau. Costello:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Usmani:Array: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Onyx: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics: Research Funding; Britsol-Myers Squibb: Consultancy, Research Funding; Skyline: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau. Berdeja:Abbvie, Acetylon, Amgen, Bluebird, BMS, Calithera, Celgene, Constellation, Curis, Epizyme, Janssen, Karyopharm, Kesios, Novartis, Onyx, Takeda, Tragara: Research Funding. Omel:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Member of Takeda's "Patient Leadership Council". Token payment. Thompson:Celgene: Membership on an entity's Board of Directors or advisory committees, Other: MDS/AML Registry; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; AIM Specialty Health: Membership on an entity's Board of Directors or advisory committees; VIA Oncology: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Multiple Myeloma International Registry; Doximity: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Shah:Array: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Schwartz:Bayer: Consultancy; Blue Cross and Blue Shield Associations: Consultancy; Pfizer: Consultancy; Takeda: Consultancy. Hajek:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Terpos:Amgen: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Genesis: Consultancy, Honoraria, Research Funding; Novartis: Honoraria. Hungria:Takeda: Consultancy; Roche: Consultancy; International Myeloma Foundation Latin America: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Speakers Bureau; Bristol: Consultancy; Amgen: Consultancy. Mateos:Takeda: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Cook:Bristol-Myers Squibb: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Glycomimetics: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Leleu:Novartis: Honoraria; LeoPharma: Honoraria; Pierre Fabre: Honoraria; Amgen: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda: Honoraria; Celgene: Honoraria; Janssen: Honoraria; TEVA: Membership on an entity's Board of Directors or advisory committees. Goldschmidt:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Seal:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Pashos:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Stull:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Romanus:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Cacioppo:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Bell:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Yu:Takeda Restricted Stock Unit (RSU), a publicly traded company: Equity Ownership; Takeda Development Center Americas, Inc., Deerfield, IL, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Luptakova:Takeda Oncology: Employment. Niculescu:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Noga:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Skacel:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Chari:Array Biopharma: Consultancy, Research Funding; Amgen Inc.: Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Pharmacyclics: Research Funding; Novartis: Consultancy, Research Funding; Janssen: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal