Abstract
Background.The overall improvement in treatment outcomes and survival of multiple myeloma (MM) patients has highlighted the persistently poor outlook for patients with del(17p). Around a third of MM patients with del(17p) are also reported to have a TP53 mutation, while this mutation is rare in the absence of del(17p). The prognostic impact of TP53 mutations in the context of del(17p) is unresolved. We investigated the presence of TP53 mutations in a cohort of del(17p) patients to correlate mutation status with clinical outcome. We assessed the types of TP53 mutations, and the timing of mutations in relation to the acquisition of del(17p).
Methods. CD138+ plasma cells were obtained from bone marrow of patients at diagnosis and prior to starting each new line of therapy, subjected to FISH analysis using standard probes, and surplus tumour cells banked. Between 2004-2015, we identified 52 patients who had at least one FISH result confirming del(17p) in ≥10% CD138+ cells, of whom 16 patients (30.8%) had del(17p) at diagnosis. In 22 patients, samples were available at more than one time point (2-4) for analysis. Clinical details, including treatment and disease response (2014 IMWG criteria) were collated. Adverse risk FISH was defined as t(4;14), t(14;16) and 1q+. Genomic DNA was extracted from CD138+ plasma cells using DNeasyBlood & Tissue Kit (Qiagen). 87 gDNAsamples were amplified by PCR and screened for TP53 mutations in exons 4-11 using DHPLC. TP53 mutations were verified by Sanger sequencing. Survival was estimated using Kaplan-Meier methods, and differences were assessed by log-rank test with p-values <0.05 considered significant.
Results. TP53 mutations were identified in 19 (36.5%) patients, and there was no significant difference in median age at diagnosis between patients with or without mutations (61.3 vs 57.3 years, p=0.07). Of the 16 patients with del(17p) at diagnosis, 5 (31.3%) had a TP53 mutation in the diagnostic sample, while 14 (38.8%) of the 36 patients in the relapse subgroup had a mutation (p=0.8).
Of the 18 TP53 variants identified, 17 were single nucleotide variants (SNV), of which 14 (82.4%) were missense mutations, 2 were nonsense mutations and 1 altered a splice site; all were documented on the IARC TP53 database. One patient had a novel in-frame indel. The majority of mutations occurred in exons 5, 6 and 8, which forms an integral part of the p53 DNA binding domain. Most mutations identified were predicted to produce a non-functional protein with less than 20% transcriptional activity, as specified by the IARC database. Eleven (57.9%) of the TP53 mutated patients also had at least one high risk genetic feature compared to 16 (48.5%) of the non-TP53 mutated patients (p=0.6).
For relapse patients, median time from diagnosis to acquisition of del(17p) was 34.5 months (range 1-178), and median time from acquisition of del(17p) to TP53 mutation was 4 months (<1-45).
In 3 patients with TP53 mutations detected at relapse, a preceding sample confirmed to harbour del(17p) was non-mutated, and occurrence of the mutation occurred at 24, 37 and 39 months after del(17p) was first detected. A further 2 patients had a sample preceding del(17p) acquisition, in which there was no TP53 mutation. For the remainder of patients, del(17p) and TP53 mutation were picked up simultaneously.
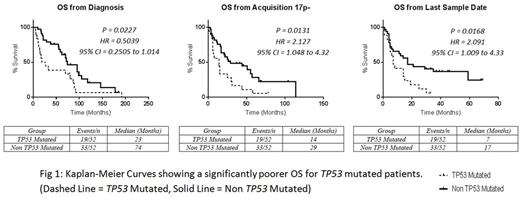
Median overall survival (OS) from diagnosis was significantly shorter for TP53 mutated patients (23 vs 74 months, p=0.03). Median OS from time of del(17p) acquisition was also significantly shorter for TP53 mutated patients (14 vs 29 months, p=0.01), although PFS from next line of therapy was similar (7 vs 12 months, p=0.70). The median survival of TP53 mutated patients from first detection of mutant TP53 was 7 months.
Summary. TP53 mutations are common in del(17p) MM with a broad range of variants, the majority of which are likely to produce non-functional variant proteins. The acquisition of TP53 mutations seems to occur after allelic loss of 17p, and to confer even worse clinical outcomes. In the era of genomic medicine, there is a case for routinely screening for TP53 mutations in these patients, to aid in management decisions and patient counselling. An urgent need exists to develop therapies to counteract the loss of this tumour suppressor pathway.
Yong:Autolus Ltd: Equity Ownership, Patents & Royalties: APRIL based chimeric antigen receptor; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal