Abstract
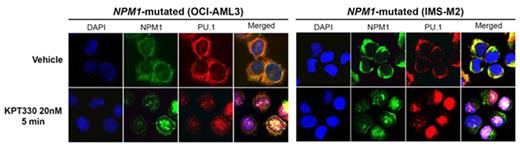
Acute myeloid leukemia (AML) is self-renewal by immature myeloid precursors that fail to differentiate. An influential 'leukemia stem cell' model thus proposes that leukemogenic proteins augment or introduce a stem cell-like self-renewal program into cells, e.g., by upregulating signaling or transcription factors (TF) emblematic of stem cells (e.g., HOX). We investigated how the most recurrently mutated protein in AML, mutant nucleophosmin (mNPM1), causes leukemic cell expansion. The results challenge this model, but most importantly, open the door to rational targeted therapy for mNPM1 AML. One way of examining for stem cell programs in AML cells is to look at expression patterns of master TF that regulate expression of hundreds of genes and dictate cell fates. Of these select TF, the master TF that create hematopoietic stem cells (HLF etc.) are minimally or not expressed. Instead, there are very high levels of the master TF that drive monocyte and granulocyte lineage fates, PU.1 (SPI1) and CEBPA. Clearly, however, the lineage-programs intended by PU.1/CEBPA are inefficiently executed if at all - mNPM1 AML patient bone marrows had 85-97% cells with a granulocyte-monocyte progenitor phenotype, accumulated at the expense of downstream mature cells (Quek et al, JEM 2016). This aggregation at a lineage-committed, intermediate, naturally proliferative level of the hematopoietic hierarchy suggests an alternative model - instead of introducing a poorly-defined stem cell self-renewal program, mutant proteins disable differentiation programs which usually quench MYC-driven proliferation intrinsic to lineage-progenitors. To better understand how mNPM1 interacts with cellular machinery, we used mass-spectrometry to comprehensively document the protein interactions of endogenous NPM1 in AML cell nuclear and cytoplasmic fractions, the first analysis of this kind. Notably, the NPM1 protein interactome was enriched for PU.1. Critically, wild-type (wt) NPM1/PU.1 was in the nucleus of wtNPM1 AML cells, but mNPM1/PU.1 was in the cytoplasm of mNPM1 AML cells. This was evident clearly also by Western blot of cell fractions and by IF microscopy of primary AML cells and cell lines. Is cytoplasmic dis-location of PU.1 sufficient to explain persistent hematopoietic precursor proliferation? We used murine Pu.1 knock-out hematopoietic precursors transduced to express Pu.1 fused with the estrogen receptor (Pu.1-ER) to answer this question - Pu.1 relocation from the cytoplasm to the nucleus by tamoxifen triggered monocytic differentiation that terminated proliferation. Moreover, Pu.1-ER cells, like mNPM1 AML cells, highly express Hox genes, rapidly suppressed upon Pu.1 relocation to the nucleus. Thus, Pu.1 dominantly controls Hox and proliferation, as befitting of a master TF, and persistent HOX expression, like persistent progenitor proliferation, can be caused by Pu.1 loss-of-function. Protein macromolecules like NPM1 require transport factors to exit (exportins) the nucleus. A specific exportin, XPO1, was the major exportin found in the NPM1 interactome. XPO1 interactions with transported cargo can be inhibited by the small molecule drug KPT330. KPT330 10-20 nM rapidly re-located mNPM1 and PU.1 to the nucleus, downregulated MYC, upregulated p27/CDKN1B, upregulated monocyte but not granulocyte differentiation markers, induced morphologic changes of monocyte differentiation, and terminated proliferation of mNPM1 AML cells. The same low nanomolar treatment did not induce differentiation of wtNPM1 AML cells (THP1). Moreover, these KPT330 levels are not toxic to normal hematopoiesis (also shown by others). Thus, rather than gain-of-function of elusive stem cell-like self-renewal, the most frequently mutated protein in AML creates self-renewal by disabling a differentiation program that quenches intrinsic MYC-driven proliferation of lineage-progenitors. These observations are a mechanistic rationale to select refractory/relapsed mNPM1 AML patients for treatment with low well-tolerated doses of KPT330, with a defined molecular pharmacodynamic objective of returning PU.1 to the nucleus, to produce cell cycle exits by differentiation rather than p53-mediated apoptosis (to address chemotherapy resistance), to spare precious normal HSC (good therapeutic index), and directly reverse the basis for leukemic self-renewal (proliferation without differentiation).
Landesman:Karyopharm Therapeutics Inc: Employment, Other: stockholder. Saunthararajah:EpiDestiny: Consultancy, Other: patents around decitabine and tetrahydrouridine.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal