Abstract
Background: For patients with smoldering multiple myeloma (SMM), the standard of care is observation. However, high-risk patients may benefit from early intervention.
Methods: In this phase 3 trial, 119 patients with high-risk SMM were randomized to treatment or observation. The high risk populationwas defined by the presence of both PC_ 10% and MC_ 3g/dl or ifonly one criterion was present, patients must have a proportionof aPC within the total PCBM compartment by immunophenotypingof 95% plus immunoparesis. Patients in the treatment group received nine 4-week induction cycles (lenalidomide at a dose of 25 mg per day on days 1 to 21, plus dexamethasone at a dose of 20 mg per day on days 1 to 4 and days 12 to 15), followed by maintenance (lenalidomide at a dose of 10 mg per day on days 1 to 21 of each 28-day cycle) up to 2 years. The primary end point was time to progression (TTP) to myeloma. Secondary end points were overall survival (OS), response rate and safety.
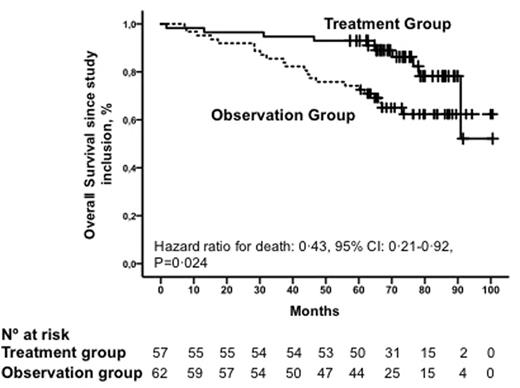
Results: After a median follow-up of 75 months (range: 57-100), there was a 57% reduction in the risk of death for the early treatment with lenalidomide-dexamethasone versus not treatment (hazard ratio, 0.43; 95% confidence interval, 0.2 to 0.9; P=0.02). Median overall survival has not been reached in either group, but 86% and 62% of patients are alive at 6 years in the early treatment and observation arms, respectively (Figure 1). The benefit in TTP is also highly sustained (hazard ratio: 0.24 (95% confidence interval, 0.14 to 0.41; P<0.0001). Progression to MM occurred in 53 out of the 62 patients (86%) in the abstention arm while only 22 out of 57 patients (38%) in the len-dex arm. At the time of progression patients received optimized treatments: bortezomib-based combinations were administered to thirteen out of 22 patients (59%) in the len-dex arm and to 23 out of 53 patients (43%) in the observation arm; lenalidomide-based combinations to 3 out of 22 patients (14%) in the experimental and to 8 out of 53 patients (15%) in the control arm; two out of 22 patients in the len-dex arm (9%) received bortezomib plus immunomodulatory agents whilst 16 out of 53 patients (30%) in the observation group received this combination; four out of 22 patients (18%) and six out of 53 patients (11%) in the len-dex and observation groups, respectively, were treated with chemotherapy; four patients (18%) in the experimental arm and 15 (28%) in the observation groups received an ASCT. Most patients responded to rescue therapies in both arms, resulting in overall response rates of 78% (17/22) and 86% (45/53) in the experimental and control arm, respectively. We compared survival from start of subsequent therapy in the patients population who progressed to active disease; the outcome was similar in both arms: at 6 years, 62% (16/22) of the patients in the len-dex arm remain alive and 49% (31/53) in the observation arm (P=0.50; Fig. 2C). The survival benefit observed was independent of the classification model used for defining high risk SMM ( Mayo Clinic and Spanish model)
Conclusion: This long term follow-up analysis confirms that early treatment with lenalidomide-dexamethasone for high-risk SMM translates into a significant benefit in TTP but also in a sustained significant prolongation of the OS. The early exposure to lenalidomide-dexamethasone does not induce more resistant relapses.
Mateos:Celgene: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Amgen: Honoraria. De La Rubia:Amgen, Bristol Myers, Celgene, Janssen: Consultancy. Paiva:Celgene: Honoraria, Research Funding; Janssen: Honoraria; Takeda: Honoraria, Research Funding; Sanofi: Consultancy, Research Funding; EngMab: Research Funding; Amgen: Honoraria; Binding Site: Research Funding. Oriol:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal