Abstract

INTRODUCTION: Frequency of NPM1 mutations in patients with myelodysplastic syndromes (MDS) is <1%. Patients with acute myeloid leukemia and NPM1 mutations have favorable outcomes and high complete response (CR) rate with chemotherapy. In this study we analyzed the characteristics and clinical outcomes of patients with MDS and NPM1 mutations.
METHODS: We analyzed the clinical characteristics of all patients with MDS and NPM1mut treated at MD Anderson Cancer Center from 2009 until 2016. Detection of exon 12 NPM1 mutations (W288fs*12) was performed by Sanger sequencing from 2009 until 2012 and by a 28 or 53 gene panel PCR-based next generation sequencing platform from 2012 to 2016. All clinical and demographic characteristics were obtained from clinical records. Response was defined following 2006 IWG criteria. The Kaplan-Meir product limit method was used to estimate the median overall survival (OS) and leukemia-free survival (LFS). Univariate Cox proportional hazards were used to identify association between variables and outcomes.
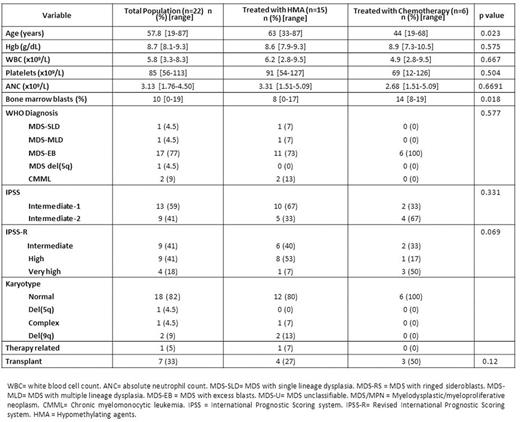
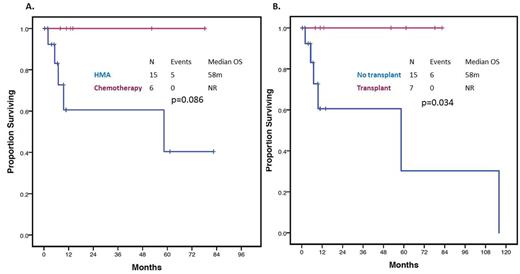
RESULTS: A total of 22 patients with MDS had detectable W288fs*12 NPM1 mutations. Patient characteristics are detailed in Table 1. Median age was 58 years (range 19-86). Thirteen (59%) patients were classified as Int-1 and 9 (41%) as Int-2 by IPSS. Two (9%) patients had CMML and the remaining had MDS. Nineteen (82%) patients had normal karyotype. All patients had available FLT3, NRAS and KIT mutational evaluation with an additional 2 (9%) patients having sequencing data on 28 genes and 11 (50%) on 53 genes. A total of 2 (9%) patients had co-occurring FLT3-ITD mutations and 1 (4.5%) had a co-occurring FLT3 D835 mutation. Other detectable mutations included NRAS in 6/22 (27%) patients, DNMT3A and WT1 in 4/13 (31%), PTPN11, TET2 and IDH2 in 2/13 (15%) and RUNX1 and IDH1 in 1/13 (8%). A total of 15 (68%) patients were treated with azacitidine or decitabine based therapy, 6 (27%) with intensive chemotherapy and 1 (4.5%) with lenalidomide. Patients treated with chemotherapy tended to be younger (44 vs 62 years, p=0.023) and have higher bone marrow blasts (14% vs 8%, p=0.018). Seven (33%) patients underwent allogeneic stem-cell transplantation: 4 of the treated with chemotherapy and 3 of the patients treated with hypomethylating agents (p=0.12). Patients treated with chemotherapy had higher overall response rates (100% vs 38%, p=0.015) and complete response rates (84% vs 13%, OR 32.5, 95% CI 2.38-443.14, p=0.006) than patients treated with hypomethylating agents. A total of 6 patients had transformation to AML. Median follow up was 10 months (range 1-115). Median OS of all the population was not reached at current follow up. Median leukemia-free survival (LFS) was not reached at current follow up. Chemotherapy was associated with a trend to improved OS compared with hypomethylating agents (NR vs 58 months, p=0.086) as shown in Figure 1A. Allogeneic stem-cell transplant was associated with improved OS irrespective of treatment (NR vs 58 months, p=0.034) as shown in Figure 1B. By univariate analysis, presence of either FLT3-ITD or D835 mutation was associated with shorter median OS (6.9 vs 115.9 months, HR = 7.42, 95% CI: 1.03-53.45, p=0.047). No other mutations were found to significantly impact outcome.
CONCLUSIONS: NPM1 mutations are extremely rare in the context of MDS and tend to appear in younger patients with excess blasts and normal karyotype. Our results suggest treatment of this subset of patients with AML-type chemotherapy followed by stem-cell transplantation could be associated with improved outcomes. Confirmation in larger studies is required.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. DiNardo:Novartis: Research Funding; Celgene: Research Funding; Abbvie: Research Funding; Agios: Research Funding; Daiichi Sankyo: Research Funding. Konopleva:Calithera: Research Funding; Cellectis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal