Abstract
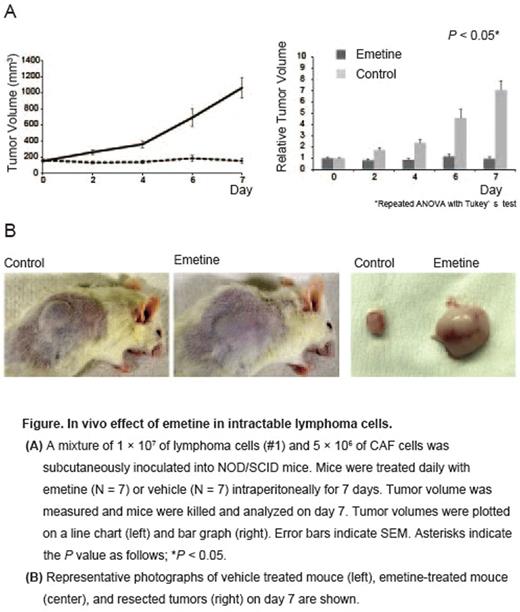
Background: Despite remarkable advances of initial treatment in diffuse large B-cell lymphoma (DLBCL), the prognosis of the disease with MYC rearrangement remains poor with a median overall survival of less than 1 year. The application of intensive or targeting treatment failed to show a benefit for the disease, an innovative approach should be thus required to overcome the obstacle of MYC rearrangement. Recent findings revealed that the close interaction of tumor cells with stromal cells in its microenvironment is involved in resistance to chemotherapy, and that tumor microenvironment has been shed light on a potential attractive therapeutic target. Purpose: To overcome poor prognoses of intractable DLBCL with MYC rearrangement, we explored an effective drug targeting tumor microenvironment through the high-throughput drug screening (Sugimoto et al. Sci Rep. 2015). Material and methods: Allpatient samples were experimentally used with written informed consent. To perform drug screening against primary patient lymphoma cells with intractable clinical course,we firstly developed co-culture system of lymphoma cells and stromal cells, which allowed us to culture them in vitro.For this, isolated stromal cells derived from human lymph node were prepared. Then 3,440 compounds mainly containing known pharmacologically active substance or off-patent drugs were screened to identify effective drugs for patient lymphoma cells. The efficacy and mechanism of action of the drug were confirmed by subsequent in vitro and in vivo analyses. Results: Two patient tumor cells with MYC/BCL2 rearrangement were used for the drug screening. Both patients developed refractory diseases within 1 year after diagnosis. In the screening analyses, primary lymphoma cells obtained from lymph node for patient (Pt) #1 were used, and tumor cells from PDX mouse model for Pt #2 were used to validate the result of Pt #1. The both tumor cells could not survive in in vitro monoculture, while the both lymphoma cells could remarkably survive longer in co-culture with stromal cells. Then we performed drug screening against primary tumor cells from Pt #1. Ninety-nine compounds with the viability of tumor cells less than 0.5 were identified, and we validated cell death of these 99 compounds against the other lymphoma cells from Pt #2. Among 10 compounds identified as potentially effective for the both tumor cells, we picked out emetine, which induced cell death against the both cells with an IC50 of 312 nM and 506 nM, respectively. Regarding the effect of emetine on stromal cells, the proliferation and survival was not affected in the concentration of 2 µM emetine whose concentration was used for the screening. However, stromal cells pretreated 0.5 µM emetine decreased a support potential to tumor cells resulting from decreased ATP production and glutathione in tumor cells. In terms of the effect of emetine on tumor cells, the drug induced a G2/M arrest in tumor cells, which resulted in induction of apoptosis. Based on previous finding that emetine suppresses HIF-1a expression, which is one of key regulators glucose metabolisms, we investigated the expression in tumor cells under the treatment of emetine. HIF-1a expression was suppressed in tumor cells as expected; we subsequently analyzed the status of glucose metabolism in tumor cells. The expression of key enzymes including HK2, PDK1, and LDHA were suppressed and ATP production and GLUT1 expression were also suppressed. The serial cascade of the alteration of glucose metabolism including the decreased mitochondrial membrane potential, the alteration of pentose phosphate pathway, and the reduction of NADPH and glutathione leading to the accrual of reactive oxygen species (ROS) was observed under the presence of emetine. In in vivo analyses, significant growth inhibition was observed under the emetine treatment (Figure A and B). Conclusions: Emetine identified by the drug screening is clearly effective for patient lymphoma cells with intractable clinical course in vitro and in vivo. Subsequent analyses regarding the mechanism of action of emetine revealed that the drug affected the both tumor cells and stromal cells in tumor microenvironment through the inhibition of glucose metabolism. Further investigations of the translation to clinic should be warranted.
Sugimoto:Otsuka Pharmaceutical Co., Ltd.: Employment. Kiyoi:Nippon Shinyaku Co., Ltd.: Research Funding; Fujifilm Corporation: Patents & Royalties, Research Funding; Eisai Co., Ltd.: Research Funding; Astellas Pharma Inc.: Consultancy, Research Funding; Phizer Japan Inc.: Research Funding; Yakult Honsha Co.,Ltd.: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding; MSD K.K.: Research Funding; Alexion Pharmaceuticals: Research Funding; Novartis Pharma K.K.: Research Funding; Mochida Pharmaceutical Co., Ltd.: Research Funding; Toyama Chemikal Co.,Ltd.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; AlexionpharmaLLC.: Research Funding; JCR Pharmaceutlcals Co.,Ltd.: Research Funding; Nippon Boehringer Ingelheim Co., Ltd.: Research Funding; Celgene Corporation: Consultancy; Zenyaku Kogyo Co.LTD.: Research Funding; Kyowa-Hakko Kirin Co.LTD.: Research Funding; Chugai Pharmaceutical Co. LTD.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal