Abstract
Background: Patients with relapsed/refractory AML have dismal outcomes with currently available chemotherapy regimens. Preclinical and clinical evidence suggests that the use of hypomethylating agents such as decitabine prior to standard chemotherapy may be synergistic (Benton et al. BJH 2014, Qin et al. CCR 2007; Scandura et al. Blood 2011).
Methods: Patients aged ≥18 to 65 years with relapsed/refractory AML (up to salvage 2) were enrolled in this investigator-initiated trial (NCT01794702). Other eligibility criteria included ECOG PS 0-2 and adequate organ function. Chemotherapy regimen consisted of Induction cycle with decitabine (20 mg/m2 IV days 1-5), clofarabine (15 mg/m2 IV days 6-9), idarubicin (10 mg/m2 IV days 6-8), and cytarabine (1 gm/m2 IV days 6-10). Consolidation cycle consisted of decitabine (20 mg/m2 IV days 1-5), clofarabine (15 mg/m2 IV days 6-8), idarubicin (8 mg/m2 IV days 6-7), and cytarabine (1 gm/m2 IV days 6-8).
Results: Fifty-Fourpatients (female, n=16) have been treated. Median age was 44 years (range 20-66). Majority of the patients (29/54, 54%) were in salvage 1. Cytogenetics were diploid (n=25), complex (n=15), CBF (n=5), 11q23 abnormality (n=2), trisomy 8 (n=2), inv 3 (n=2), misc (n=3). Six patients had a TP53 mutation and 6 patients had an NRAS mutation. Seven patients had NPM1 mutation. No patient had a FLT3 ITD or D835 mutation. Median number of cycles administered was 1 (range 1-3). Grade 3 mucositis was seen in 2 patients and grade 3 transaminitis in 8 patients. Thirty-three patients had one or more infections. Early deaths occurred in 4 (7%) patients within 4 weeks, and 8 (15%) patients within 8 weeks.
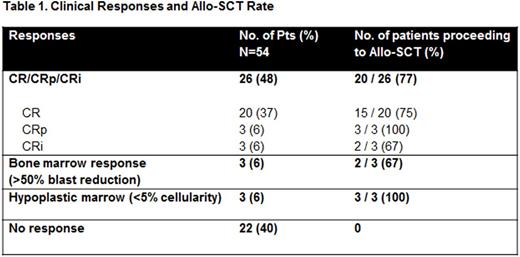
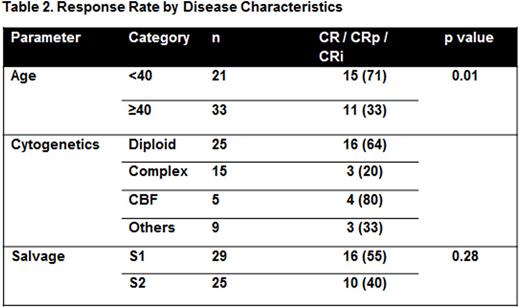
Twenty six (48%) patients achieved CR/CRi (Table 1). Median time to CR was 42 days. An additional 3 patients had a >50% decrease in bone marrow blast count (bone marrow response) and an additional 3 patients had aplastic/hypoplastic marrow at the end of induction. Patients younger than 40 years had a significantly increased likelihood of achieving a CR/CRp/CRi (71% vs. 33%, p = 0.01) (Table 2). Eighty percent of patients with CBF AML and 64% of patients with diploid cytogenetics achieved a CR/CRp/CRi. CR rate in patients with complex cytogenetics was 20%.
Twenty-five (46%) patients underwent allo-SCT (20 CR/CRp/CRi, 2 with 50% marrow blast reduction, 3 with aplastic/hypoplastic marrow). 13/21 (62%) patients younger than 40 years of age were able to undergo allo-SCT as compared to 12/33 (36%) of patients 40 years of older (p = 0.09).
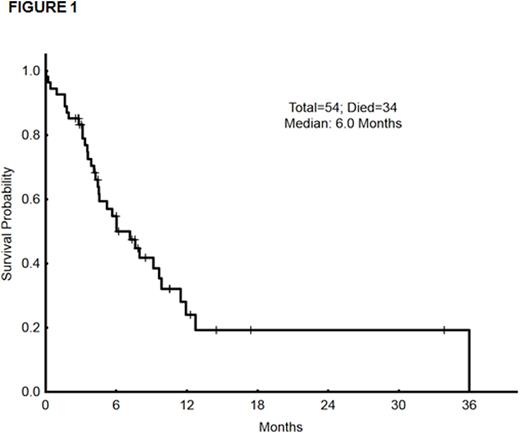
Ten patients have relapsed (including six after allo-SCT). Twenty patients are alive (13 after stem cell transplant, 1 is still on study, 4 are receiving further salvage regimens after DAC-CIA failure, 2 lost to follow-up). Median survival of the entire group is 6.0 months (figure 1).
Conclusions: The sequential treatment of decitabine followed by chemotherapy is safe and effective with a CR/CRp/CRi rate of 48% and with 25/54 (46%) patients able to proceed to an allo-SCT.
Jain:Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria; Incyte: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Seattle Genetics: Research Funding; Novimmune: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Infinity: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Abbvie: Research Funding; BMS: Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Daver:Kiromic: Research Funding; BMS: Research Funding; Pfizer: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Otsuka: Consultancy, Honoraria; Ariad: Research Funding; Karyopharm: Honoraria, Research Funding. DiNardo:Novartis: Other: advisory board, Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Abbvie: Research Funding; Celgene: Research Funding; Agios: Other: advisory board, Research Funding. Konopleva:Reata Pharmaceuticals: Equity Ownership; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Stemline: Consultancy, Research Funding; Eli Lilly: Research Funding; Cellectis: Research Funding; Calithera: Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal