Abstract
Background: Patients (pts) aged >65 years (yrs) with AML have a median overall survival (OS) of ~3 months (mos), with worsening OS as age increases: median OS for pts ages 66-75 yrs is ~6 mos, but is only ~2.5 mos for pts ages 76-89 yrs (Walter, Leukemia, 2015). Treatment (Tx) options are limited for these pts, who often have disease features associated with Tx resistance, such as prior hematologic disorders, and a greater risk of Tx-related mortality. With current Tx options, in this setting many physicians or pts do not pursue active AML Tx. The phase 3, randomized AZA-AML-001 study compared AZA and CCR in older pts with AML (Dombret, Blood, 2015).
Aims: Assess outcomes with AZA vs CCR in elderly pts (age ≥75 yrs) with AML in the AZA-AML-001 study overall and in the subset of pts with myelodysplasia-related changes (AML-MRC), and descriptively compare them with outcomes for pts aged 65-74 yrs.
Methods: Pts aged ≥65 yrs with newly diagnosed AML (>30% bone marrow blasts), ECOG performance status ≤2, intermediate- or poor-risk cytogenetics, and WBC counts ≤15x109/L were eligible. Pts were randomized to receive AZA (75 mg/m2/day [d] x7d/28d) or CCR: low-dose ara-C (20mg SC BID x10d/28d), intensive chemotherapy (7+3), or best supportive care only. These analyses evaluated outcomes in pts aged 65-74 yrs ("<75 yrs") and pts aged ≥75 yrs. WHO-defined AML-MRC was centrally confirmed. OS was estimated using Kaplan-Meier (KM) methods, with hazard ratios (HR) and 95% confidence intervals (95%CI) from an unstratified Cox proportional hazards model and P values from log-rank test. Survival at 3, 6, 9, and 12 mos was estimated using KM methods. Overall response rate (ORR) included complete remission (CR) and CR with incomplete hematologic recovery (CRi). Incidence rates (IRs; ie, rate normalized for Tx exposure) of grade 3-4 Tx-emergent adverse events (TEAEs) and infections leading to death per 100 pt-years of Tx exposure are reported for safety-evaluable pts (those who received ≥1 dose of study drug [at randomization for BSC only] and had ≥1 safety assessment post-dose).
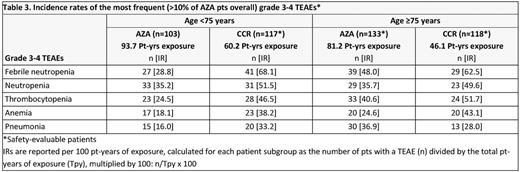
Results: In all, 223 pts were aged <75 yrs (AZA n=103; CCR n=120) and 265 were aged ≥75 yrs (AZA n=138; CCR n=127), including 27 pts aged >85 yrs (AZA n=14; CCR n=13). Median age in the <75 yrs cohort was 71 yrs, and in the ≥75 yrs cohort was 78 and 79 yrs for the AZA and CCR groups, respectively (Table 1). Median OS was meaningfully prolonged with AZA vs CCR in pts aged <75 yrs (14.2 vs 9.6 mos; HR 0.73, 95%CI 0.54, 0.99; P=0.0420). OS was also prolonged with AZA in pts aged ≥75 yrs vs CCR, but was not statistically different (median 7.0 vs 4.9 mos; HR 0.91, 95%CI 0.69, 1.2; P=0.46). Higher proportions of AZA-treated pts were alive at each 3-mo landmark in both age cohorts (Table 2). ORR was similar with AZA and CCR in pts ages <75 yrs (32% and 30% respectively) and ≥75 yrs (25% and 21%), with a trend for a higher rate of CRi in AZA-treated pts aged ≥75 yrs (8% vs 2%; P=0.054). In the AML-MRC subgroup (n=262), median OS in pts aged <75 yrs was meaningfully prolonged with AZA (n=52) vs CCR (n=64) (14.2 vs 7.3 mos, respectively; HR 0.64, 95%CI 0.42, 0.97) and nominally so with AZA (n=77) vs CCR (n=69) in pts ≥75 yrs (5.9 vs 3.8 mos; HR 0.77, 95%CI 0.54, 1.09). IRs of the most frequent grade 3-4 hematological TEAEs were lower with AZA vs CCR in the both age cohorts (Table 3), except the IR for grade 3-4 pneumonia in pts aged ≥75 yrs was higher with AZA. IRs of infections leading to death in the AZA and CCR groups were 14.9 and 38.2 per 100 pt-yrs, respectively, for pts aged <75 yrs, and 33.3 and 28.0 per 100 pt-yrs in pts aged ≥75 yrs.
Conclusions: As expected, OS and response rates were lower in elderly pts in both Tx arms than in younger pts. Median OS was meaningfully prolonged with AZA (+4.6 mos) vs CCR in pts aged 65-74 yrs. Higher proportions of AZA-treated pts remained alive at each 3-month landmark than CCR-treated pts, mainly in the younger age group, although 1-year survival was also higher in pts aged ≥75 yrs. Given the higher IR of infections, prophylactic use of antimicrobials or growth factors might be considered for elderly pts treated with AZA. Pts with AML-MRC retained the relative OS benefits of AZA vs CCR. While OS for AML-MRC pts aged ≥75 yrs was longer with AZA (+2.1 mos) vs CCR, pts with AML-MRC aged ≥75 yrs in both treatment groups had decreased OS compared with the median OS for all pts aged ≥75 yrs, consistent with reports that AML-MRC is more difficult to treat than AML not otherwise specified (Weinberg, Blood, 2009).
Seymour:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Buckstein:Celgene: Honoraria, Research Funding; Novartis: Honoraria. Santini:Astex: Consultancy; Onconova: Consultancy; Amgen: Consultancy; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding. Stone:Seattle Genetics: Consultancy; Jansen: Consultancy; Amgen: Consultancy; Novartis: Consultancy; ONO: Consultancy; Agios: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy; Pfizer: Consultancy; Juno Therapeutics: Consultancy; Xenetic Biosciences: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy; Roche: Consultancy; Sunesis Pharmaceuticals: Consultancy; Celator: Consultancy. Songer:Celgene: Employment, Equity Ownership. Weaver:Celgene Corporation: Employment, Equity Ownership. Beach:Celgene Corporation: Employment, Equity Ownership. Dombret:Agios: Honoraria; Sunesis: Honoraria; Ambit (Daiichi Sankyo): Honoraria; Karyopharm: Honoraria; Kite Pharma.: Honoraria, Research Funding; Menarini: Honoraria; Menarini: Honoraria; Astellas: Honoraria; Janssen: Honoraria; Servier: Honoraria; Seattle Genetics: Honoraria; Roche/Genentech: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Honoraria; Ariad: Honoraria, Research Funding; Novartis: Honoraria; Celgene: Consultancy, Honoraria; Jazz Pharma: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal