Abstract
Introduction: T-cell ALL and T-LL are considered as different spectra of the same neoplastic clone. In various clinical trials of adult ALL, patients with T-ALL and T-LL were combined when analyzing treatment responses and survival outcomes. We have previously reported the results with HCVAD-based regimens in patients with adult ALL. In this study we addressed whether the initial presentation, treatment response, and survival outcomes of adults with T-LL and T-ALL differed when patients were uniformly treated with frontline HCVAD-based regimens at a single institution.
Methods: One hundred and fifty previously untreated patients with T-LL (n=54) and T-ALL (n=96) who were treated with HCVAD-based regimens (1992-2016) were included in this analysis. Patient charts were reviewed for initial characteristics, treatment responses including minimal residual disease (MRD) status and survival outcomes; event free (EFS) and overall survival (OS) were analysed.
Results: Among 150 patients with previously untreated adult T-ALL/LL, we identified 54 patients (36%) with T-LL and 96 (64 %) with T-ALL. Among patients with available immunophenotype data (n=104), early T precursor (ETP) phenotype was significantly more frequent among patients with T-ALL compared to patients with T-LL (44% vs 19%; p=0.006). The proportion of early, cortical and mature immunophenotype were 2% vs 6%, 31% vs 49% and 16% vs 12% in T-ALL versus T-LL, respectively. The clinical characteristics, response to therapy and outcomes of patients in T-LL versus T-ALL were compared (Table 1 and Figure-1).
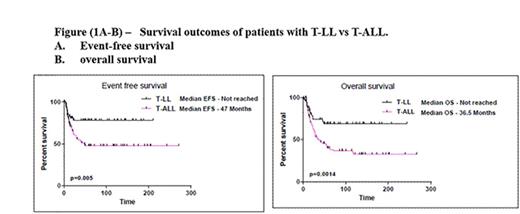
Patients with T-ALL were slightly older at presentation (median age 37 years [18-67] versus 31 years [17-78]; p=0.07). Patients with T-ALL had significantly higher white blood cell counts, peripheral blood blasts %, bone marrow blasts %, and serum LDH as compared to patients with T-LL. Distribution of chromosomal aberrations was significantly different among the two groups: Diploid karyotype was more commonly encountered in patients with T-LL while patients with T-ALL had more hyperdiploidy and hypodiploidy. Among patients evaluable for response, complete remission (CR) rates were 85% and 95% (p=0.002) in T-LL and T-ALL, respectively. Overall the median follow up times were 72 months (range, 5-243) and 61 months (range, 1-267). Thirty nine (72%) patients with T-LL and 43 (45%) with T-ALL were alive at the time of last follow-up. Patients with T-LL had better outcomes than patients with T-ALL. The 3-year EFS and OS rates were 78% and 74% in patients with T-LLand 53% (p=0.005) and 50% (p=0.001) in patients with T-ALL (Figure 1).
Conclusions: In summary, adult patients with T-LL have better outcomes than patient with T-ALL after treatment with HCVAD-based regimens. Additional studies to characterize the genomic profile in tumoral tissues, as well as the pattern of relapses in patients with adult T-LL and T-ALL are ongoing.
Summary of patient characteristics according to initial diagnosis - T-lymphoblastic lymphoma (T-LL) vs. T-acute lymphoblastic leukemia (T-ALL)
*104 patients had full immunophenotype for classification, nos - not otherwise specified, **On available cytogenetic data (47 in T-LL and 82 in T-ALL), of note 3 patients in T-LL and 22 in T-ALL have miscellaneous chromosomal abnormalities
Summary of patient characteristics according to initial diagnosis - T-lymphoblastic lymphoma (T-LL) vs. T-acute lymphoblastic leukemia (T-ALL)
*104 patients had full immunophenotype for classification, nos - not otherwise specified, **On available cytogenetic data (47 in T-LL and 82 in T-ALL), of note 3 patients in T-LL and 22 in T-ALL have miscellaneous chromosomal abnormalities
Konopleva:Calithera: Research Funding; Cellectis: Research Funding. Jain:Incyte: Research Funding; Servier: Consultancy, Honoraria; Seattle Genetics: Research Funding; Infinity: Research Funding; Novimmune: Consultancy, Honoraria; Abbvie: Research Funding; Celgene: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Genentech: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; BMS: Research Funding. Wierda:Acerta: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Gilead: Research Funding; Genentech: Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. O'Brien:Janssen: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal