Abstract
Background: Sickle cell disease (SCD) is caused by polymerization of Hemoglobin S (HbS), resulting in hemolysis and vaso-occlusion. No therapy achieving pancellular, direct inhibition of HbS polymerization is available. GBT440 is a novel small molecule which increases hemoglobin oxygen affinity and inhibits HbS polymerization and prevents sickling in vitro. This study explored safety, pharmacokinetics (PK) and pharmacodynamics (PD) of GBT440 in healthy volunteers (HV) and subjects with SCD.
Methods: This randomized, placebo-controlled, double-blind, phase I/II study enrolled HV and SCD subjects (HbSS and HbSB0). A cohort of 8 subjects with other SCD genotypes (HbSC or HbS/β+thalassemia) is being enrolled. The study was conducted in three parts: Part A, single ascending doses, Part B, multiple ascending doses for 28 days and Part C, 90-day dosing. Longer term dosing (up to 6 months) is being evaluated. The primary endpoint was safety. Secondary endpoints included PK, PD and hematological effects.
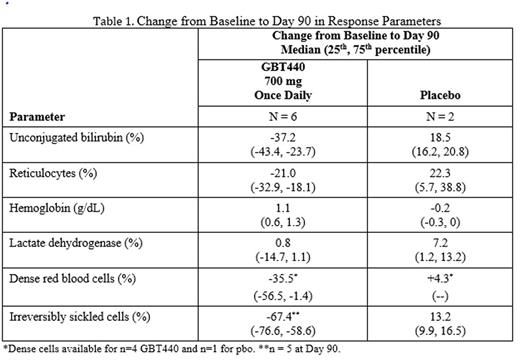
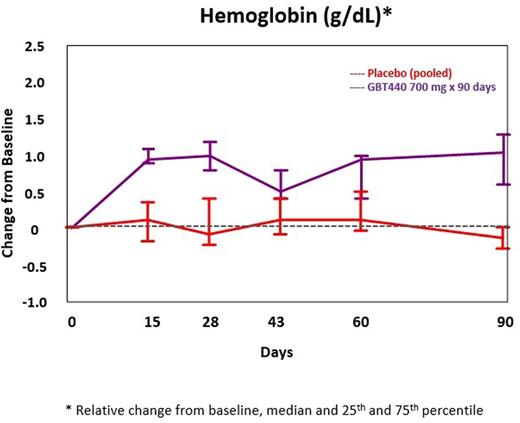
Results: New and updated results including up to 6 months of dosing and data on other SCD genotypes will be presented at the meeting. As of 26 July 2016, 38 SCD subjects had completed Part B (10 at 500 mg; 12 at 700 mg; 6 at 1000 mg; 10 received placebo [pbo]). An additional 16 SCD subjects have completed or are ongoing in Part C (6 at 700 mg: 6 at 900 mg; 4 on pbo). Enrollment is ongoing for SCD subjects with HbSC or HbS/β+thalassemia. Results are available for SCD subjects enrolled into Part B and Part C (700 mg). The SCD subjects include approximately 60% male, 40% female; 20% were on hydroxyrea (HU); 20% had 2 or more painful crises requiring hospitalization in the prior year; median age was 34 years (range, 20 to 56). There were no drug-related severe or serious adverse events; the majority of AEs were Grade 1 or 2. The most common treatment emergent AEs were headache, back pain and sickle cell crisis. All sickle cell crises on placebo or GBT440 occurred off treatment and were assessed as not related to study drug. PK exposures increased dose-proportionally; mean GBT440 whole blood half-life following multiple doses was approximately 1.5 days and RBC:plasma ratio was 75:1. GBT440-treated subjects demonstrated increased hemoglobin oxygen affinity (p50 shifted to the left towards the normal range), an increase in hemoglobin levels and a sustained decrease in unconjugated bilirubin during the 90 day treatment period (Figure 1, Table 1). Other markers of hemolysis improved concordantly (Table 1).
Conclusions: GBT440 was well tolerated with dose-proportional PK and increases in hemoglobin oxygen affinity. GBT440 resulted in marked and sustained reduction in clinical markers of hemolysis and an increase in hemoglobin. These results are consistent with inhibition of HbS polymerization leading to decreased RBC damage, improved RBC lifespan and tissue oxygen delivery, and support further investigation of GBT440 as a potential disease-modifying therapy for SCD.
Change from Baseline to Day 90 in Hemoglobin (GBT440 700 mg)
Lehrer-Graiwer:Global Blood Therapeutics: Employment, Equity Ownership. Mant:Quintiles: Employment. Dufu:Global Blood Therapeutics: Employment, Equity Ownership. Hutchaleelaha:Global Blood Therapeutics: Employment, Equity Ownership. Oksenberg:Global Blood Therapetics: Employment, Equity Ownership. Patel:Global Blood Therapeutics: Employment, Equity Ownership. Tonda:Global Blood Therapetics: Employment, Equity Ownership. Bridges:Global Blood Therapetics: Employment, Equity Ownership. Ramos:Global Blood Therapeutics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal