Abstract
Beta thalassemia is an inherited hemoglobinopathy due to reduced synthesis of Beta globin chains and, consequently, of hemoglobin A (a2b2). The clinical manifestations are mainly the result of chronic anemia and iron overload. The latter is due to increased iron absorption, induced by accelerated but ineffective erythropoiesis, and recurrent red blood cell transfusions. Alfa-chains and iron excess promote oxidative damage of red blood cell membrane, resulting in macrophage sequestration and extravascular hemolysis, and to a lower extent, in intravascular hemolysis, with consequent release of hemoglobin (Hb), heme and iron.
Increasing evidence suggests that free heme exerts vasculotoxic, pro-inflammatory and procoagulant effects due to its ability to trigger endothelial and immune cells activation. In addition, a role for heme and iron has been postulated in the pathogenesis of other vascular diseases, including atherosclerosis. In mouse models of Beta thalassemia and sickle cell disease, circulating heme levels are elevated and correlate with the exhaustion of systemic scavengers for hemoglobin and heme, haptoglobin and hemopexin, respectively, as well as with severe endothelial dysfunction and inflammation. Hemopexin-based therapies significantly improve endothelial damage, vascular oxidative stress and inflammation in these mice (Vinchi et al., Circulation 2013, Blood 2016; Vercellotti GM. et al., Mol Med 2016).
Whereas more data are reported on sickle patients in this regard, few data are available in patients with Beta thalassemia. In the present study, we examined serum samples from a cohort of 60 patients with Beta thalassemia major (age 11.5 ± 6.8, 44% males-56% females, Hb 7.69 ± 1.22 mg/dl, transfused every 3-4 weeks) and 7 patients with Beta thalassemia intermedia (age 14 ± 12 , 70% males-30% females, Hb 8.4 ± 0.74 mg/dl, transfused every 4-5 weeks). 10% of the patients received inconsistent iron chelation therapy. Serum from 10 healthy subjects (age 22.7±15.3, 50% males-50% females, Hb 13.12±1.15 mg/dl) served as control.
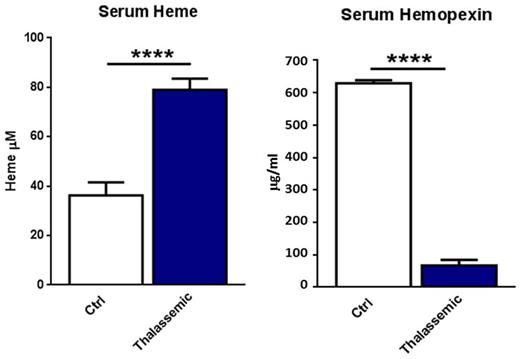
Both groups of patients show high systemic heme and iron levels, which associate with a severe drop in serum haptoglobin, hemopexin and transferrin. Consistently, transferrin saturation (12.4±2 vs 79.6±24 %) and serum ferritin (55.14 ±0.23 vs 4919.2 ±2657.4 ng/ml) are elevated. Interestingly, these patients present with high systemic levels of the soluble adhesion molecules sVCAM-1 and sICAM-1, markers of enhanced endothelial activation. In addition, they show increased levels of serum malondialdehyde, a well-known marker of lipid peroxidation and oxidative stress, and high levels of circulating oxidized low density lipoproteins (oxLDL). All parameters significantly correlate with increased systemic heme and iron indices as well as decreased haptoglobin, hemopexin and transferrin levels.
In conclusion, Beta thalassemia patients show a strong correlation between systemic heme and iron overload, depletion of the respective scavengers, and markers of oxidative stress and endothelial dysfunction, thus confirming studies in animal models. These results emphasize the involvement of serum hemoglobin, heme and iron in the pathophysiology of Beta thalassemia, including vascular dysfunction, and the key protective role of their carriers. These findings are relevant for disorders hallmarked by vasculopathy, such as sickle cell disease and Beta thalassemia, as well as cardiovascular diseases, such as atherosclerosis. Our data support the potential therapeutic benefit of the administration of hemoglobin/heme scavengers along with efficient iron chelation therapy to counteract heme- and iron-driven toxicity.
(The last three authors equally contributed to the work)
****P<0.0001
Vercellotti:CSL-Behring: Research Funding; Imara: Research Funding. Belcher:Cydan/Imara: Research Funding; CSL-Behring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal