Abstract
Introduction:Regular supportive transfusion therapy from an early age is common practice in young children with β thalassemia major (βTM), sickle-cell disease (SCD) and Diamond-Blackfan anemia (DBA). Patients consequently accumulate iron that can affect organ function and delay growth/development. As such, iron chelation therapy is often necessary from an early age, with lifelong requirements for patients who cannot be cured with hematopoietic stem cell transplantation. Deferasirox, a once-daily oral iron chelator, has demonstrated efficacy and safety in adult and pediatric patients with chronic iron overload. Long-term evaluation of deferasirox in young pediatric patients with transfusional hemosiderosis is valuable, as conducted in the 5-yr multinational, observational ENTRUST study. Here, we report safety and efficacy outcomes in pediatric patients with transfusional hemosiderosis receiving up to 5 yrs of continuous deferasirox treatment in clinical practice.
Methods:Patients aged 2-<6 yrs at enrolment were prescribed deferasirox according to local labels, with recommendations to adjust dose based on serum ferritin levels, therapeutic goals, tolerability and weight gain. Patients were followed prospectively for 5 yrs in this observational study. Safety was evaluated by regular monitoring and recording of adverse events (AEs) in all patients who received ≥1 dose of deferasirox and had ≥1 post-baseline safety assessment (safety set). Serum ferritin levels were also analyzed (as a surrogate marker for body iron) in all patients who received ≥1 dose of deferasirox (full analysis set; FAS).
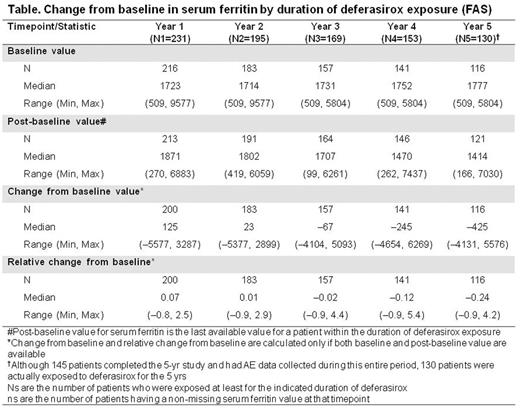
Results:267 patients (mean age 3.2 yrs: 61.4% <4 yrs, 38.6% ≥4 yrs) with βTM (n=176, 65.9%), SCD (n=52, 19.5%), DBA (n=12, 4.5%), and other anemias (n=27, 10.1%) were enrolled and received ≥1 deferasirox dose. Overall mean ± SD deferasirox exposure was 44.1 ± 21.2 (range 1.2-65.6) months and mean ± SD dose was 25.8 ± 6.5 mg/kg/day. Dose was generally aligned with weight, though initial doses were suboptimal to manage iron intake and adjustments based on weight gain were delayed. 130 patients (49.8%) received deferasirox for ≥60 months. 122 patients (45.7%) patients discontinued treatment prematurely, most commonly (>5% of patients) because of loss to follow-up (n=19, 7.1%) and AEs (n=18, 6.7%; most commonly increased alanine or aspartate aminotransferase [ALT/AST], n=7 each). The number of patients discontinuing treatment because of AEs declined year on year; n=10 in Yr 1, n=4 in Yr 2, n=2 in Yr 3, and n=1 in Yrs 4 and 5, respectively. From Yr 3 onwards, there were no discontinuations due to an increase in ALT or AST. Change from baseline in serum ferritin by duration of deferasirox exposure (FAS) is summarized (Table). In the 130 patients exposed to deferasirox for 5 yrs, median serum ferritin levels decreased from 1777 (range 509-5804) ng/mL at baseline to 1414 (range 166-7030) ng/mL, a median change from baseline of -425 (range -4131-5576) ng/mL (Table).
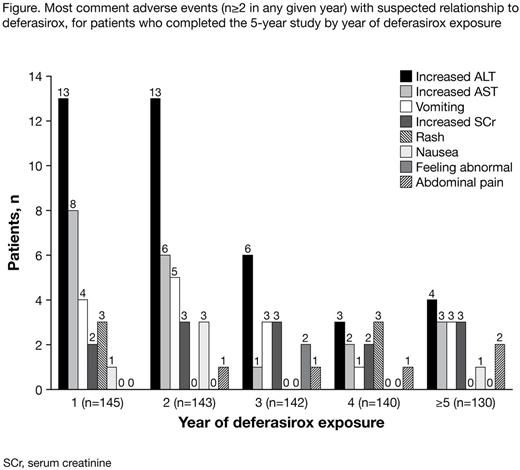
145 patients completed 5 yrs of follow-up and had data on AEs collected during this entire period. The most frequently occurring AEs with suspected relationship to study drug (occurring in ≥4 patients) were increased ALT (n=29, 20.0%), increased AST (n=12, 8.3%), vomiting (n=9, 6.2%), rash (n=6, 4.1%) and increased blood creatinine (n=6, 4.1%). Overall, there was a gradual decrease in the number of patients experiencing drug-related AEs in the safety set with each year of deferasirox exposure (Figure).
Conclusions:This long-term, observational study of deferasirox in pediatric patients supports previous findings indicating favorable safety and efficacy. Approximately half the patients completed the 5-yr study, with many patients discontinuing for reasons unrelated to study drug, indicating good acceptance of treatment. Patients remaining on deferasirox benefited from sustained improvements in iron load, though this was not immediate, likely because of delayed increases in dose based on weight gain and ongoing iron intake. Among patients who continued on deferasirox tolerance was increasing over time as indicated by yearly decreasing numbers of AEs. In patients followed for up to 5 yrs, AEs were consistent with the known deferasirox safety profile, with no progressive increases in the incidence of renal-related AEs. These data therefore support the long-term use of deferasirox in young iron overloaded patients in everyday clinical practice.
Vichinsky:Novartis, ApoPharma Inc. and ARUP Laboratories: Research Funding; Novartis: Consultancy. El-Beshlawy:Novartis, ApoPharma Inc: Research Funding. Bruederle:Novartis Pharmaceuticals Corporation: Employment. Han:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal