Abstract

INTRODUCTION: Alterations of innate immune signaling, including overexpression of TLR2, are common in MDS. Signaling mediated by this receptor results in activation of NF-kB and the histone demethylase JMJD3 (KDM6B) leading to expression of multiple cytokines that can result in inhibition of hematopoiesis. Significant TLR2 overexpression in MDS bone marrow CD34+ cells, especially after HMA therapy, has been reported (Wei, Leukemia 2013). OPN-305 is a fully humanized antagonistic IgG4 kappa monoclonal antibody to TLR2 which significantly increases the formation of erythroid colonies (CFU-E) in BM CD34+ cells isolated from pts with lower-risk MDS in vitro. This data suggested the potential therapeutic value of OPN-305 in patients (pts) with MDS.
METHODS: We designed a phase I/II trial of OPN-305 for pts with Low or Int-1 risk MDS by IPSS after failure to prior therapy with a HMA. Pts with isolated del(5q) should have received therapy with lenalidomide. Pts with prior history of AML or alloSCT were excluded from the study. Because, OPN-305 had not been previously used in pts with hematological malignancies, the study had an initial phase of N=10 pts using OPN-305 at a dose of 5 mg/kg every 4 weeks for a maximum of 9 cycles. Therapy could be repeated as long as there was no excess toxicity or progression. If after 16 weeks of therapy, there was no response, azacitidine on a 3 day schedule, could be added to OPN-305. Responses were evaluated following the revised 2006 IWG criteria. This initial cohort allowed evaluation of toxicity, pharmacokinetic analysis, receptor occupancy, and sequential analysis of cytokine profile. Depending on results, dose escalation to 10 mg/kg monthly could be instituted in a second cohort of N=5 pts.
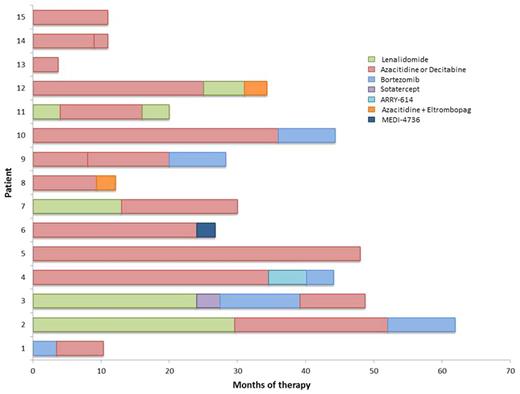
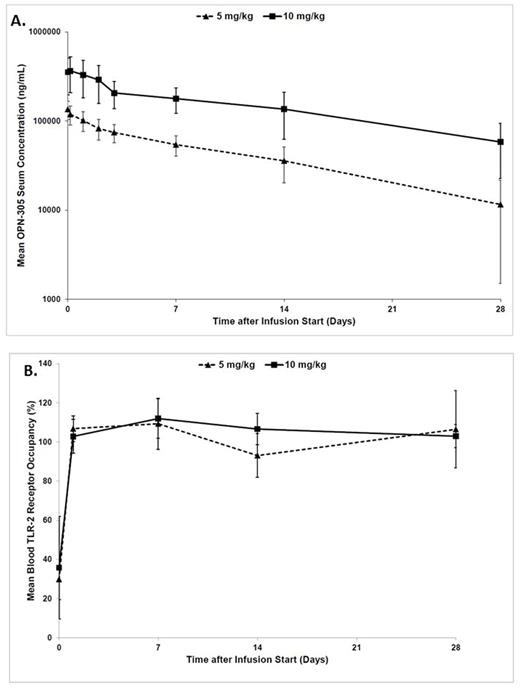
RESULTS: At the time of this report, 15 pts have been enrolled, 11 at the initial 5 mg dose and 4 at 10 mg/kg. All pts were evaluable for toxicity and 12 (80%) were evaluable for response. Patient characteristics are shown in Table 1. Median age was 73 years (range 48-87). Three (20%) pts were classified as Low risk and 12 (80%) as Intermediate-1 risk by IPSS. Seven pts had normal karyotype, 2 del(5q), 2 trisomy 8, 1 del(20q), 1 monosomy Y and 2 other single or double abnormalities. Median number of prior therapies was 2 (range 1-4) with a median duration of prior therapies of 28 months (range 4-62) (Figure 1). A total of 13 (87%) pts were transfusion dependent of red cells prior to enrollment. Median follow up is 8 months (1-16). Median number of cycles administered is 5 (2-17) with 5 (33%) pts having received addition of azacitidine after 16 weeks of therapy. A total of 5 (42%) pts developed AEs related to OPN-305. All AEs were grade 1 with gastrointestinal disorders being the most frequent (33%). At this point, no significant drug related toxicity has been documented with no excess infectious complications. Overall response rate in the form of hematological improvement was 50% (6/12) with 2 (17%) pts achieving transfusion independence. A total of 5 (33%) pts remain on study with 3 pts continuing on therapy off study. Three (20%) pts were taken off study due to progression to AML and 4 (27%) due to no response. Half-lives of OPN-305 in serum were >200 h at 5 mg/kg and >300 h at 10 mg/kg (Figure 2A). There was a greater-than-dose proportional increase in mean OPN-305 exposure (AUC) between 5 and 10 mg/kg. PK profiles after repeated dosing at 5 mg/kg in N=2 subjects and pre-dose (trough) levels in other subjects indicated some variability in the potential for accumulation. TLR-2 receptor occupancy in blood PBMCs and bone marrow aspirates was essentially complete in virtually all samples taken after OPN-305 administration (Figure 2B). There is no evidence of treatment related anti-drug antibodies. Compared with baseline, no statistically significant dynamic changes of IL-23, IL-18, IFN-r, IL-10, IL-1β, IL-6, IL-12 (p40), IL-12 (p70) and IL-8 levels where observed after treatment, among responders or non-responders or based on OPN-305 dosing.
CONCLUSIONS: Treatment with OPN-305 in pts with previously treated lower-risk MDS was well tolerated with no significant toxicities and 50% ORR. Correlative studies suggest adequate receptor occupancy with no correlation between responses and cytokine levels. Because no effect on cytokine profile, we plan to continue to escalate the dose of OPN-305 and investigate its activity profile in front line therapy.
DiNardo:Daiichi Sankyo: Research Funding; Agios: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Celgene: Research Funding. Konopleva:Reata Pharmaceuticals: Equity Ownership; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Stemline: Consultancy, Research Funding; Eli Lilly: Research Funding; Cellectis: Research Funding; Calithera: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal