Abstract
Novel agents have improved outcomes in MM, but prognosis after patients relapse remains poor and new drugs with novel MoA are needed. The breakthrough of immuno-oncology has rendered new therapeutic options, and most recently we reported on EM801, a novel BCMA-TCB that showed remarkably efficacy when used as single agent in primary bone marrow (BM) samples from MM patients (Seckinger, Blood 2015;126: abstr 117). Because of its novelty, further knowledge about the MoA of BCMA-TCB is of utmost importance to improve its efficacy by designing rational treatment combinations.
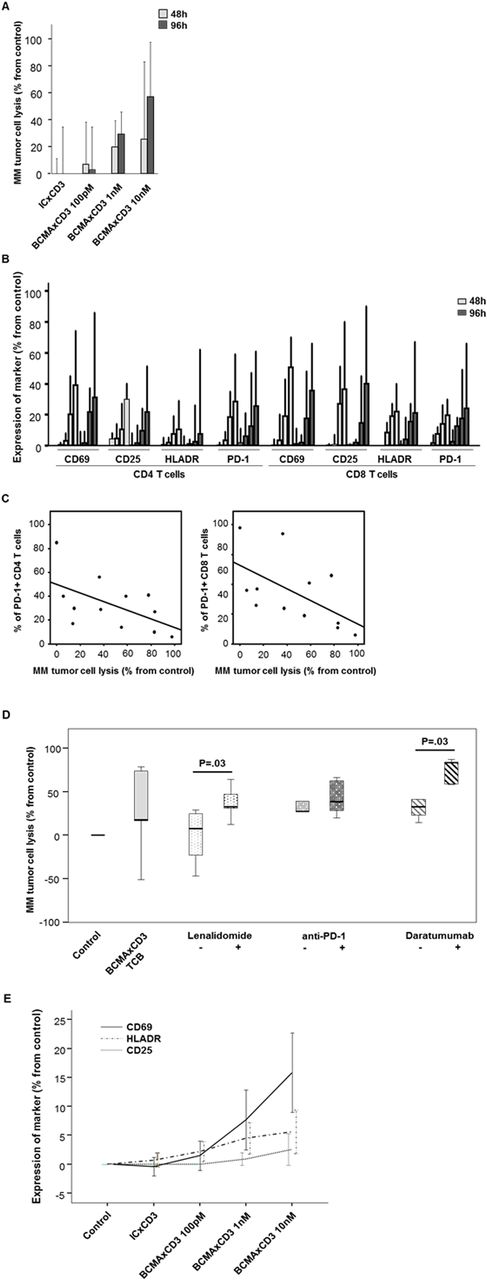
In order to optimize the in vitro efficacy of the BCMA-TCB, we started by investigating in primary BM samples from 6 MM patients whether longer treatment periods with BCMA-TCB2 (a BCMA-TCB candidate sharing similar "2+1" structure of EM801 but displaying higher affinity to BCMA) would increase MM cell death. Upon treating samples with BCMA-TCB2 for 48h vs 96h, we noted a 2-fold increment in MM tumor cell lysis at 1nM and 10nM concentrations (Panel A). In parallel, the phenotypic profiles of CD4 and CD8 T cells showed that BCMA-TCB2 induced robust activation (ie. dose-dependent increment in CD69, CD25, HLADR after exposure to 100pM, 1nM and 10nM of BCMA-TCB2), but also led to the natural emergence of the checkpoint inhibitor PD-1 in the surface of activated CD4 and CD8 T cells (Panel B). We then investigated if there was a correlation between the percentage of PD-1 positive CD4 and CD8 T cells and the efficacy of BCMA-TCB2; interestingly, those patients with lower frequencies of PD-1 positive CD4 and CD8 T cells prior to treatment showed the highest rates of MM tumor cell lysis after 48h and 96h of BCMA-TCB2 at 10nM of (r=0.6, P=0.04; Panel C). By contrast, upon measuring the concentration of soluble BCMA and APRIL in the supernatants of primary BM samples from 16 MM patients treated with BCMA-TCB, we found no significant differences between responding (n=11) and non-responding (n=5) patients. Similar results were observed upon comparing the density of BCMA in the surface of MM tumor cells from responding vs non-responding patients (1256 vs 1522 SABC units; P=87).
Since the efficacy of BCMA-TCB2 was found to be intrinsically related to the phenotype and activation status of T cells, we then investigated whether we could further harness immune cells by combining BCMA-TCB2 with three drugs representing different types of immunotherapy: lenalidomide (IMIDs), anti-PD1 (checkpoint inhibitors) and daratumumab (mAb). H929 MM cells were co-cultured with human leukocytes (n=5) and challenged to suboptimal concentrations of BCMA-TCB2 (10pM) alone, or in combination with standard doses of lenalidomide (1µM), anti-PD1 (10µg/ml) and daratumumab (10µg/ml) (Panel D). Interestingly, we observed that combining BCMA-TCB2 with lenalidomide or daratumumab significantly increased their anti-MM efficacy by 4-fold and 2.5-fold, respectively. Because lenalidomide and daratumumab share in common that they rely, at least in part, on activated NK cells to eradicate MM cells, we hypothesized whether such robust T cell activation induced by BCMA-TCB2 was leading to co-stimulation of NK cells. First, we demonstrated by analyzing the transcriptomes of T cells prior and after treatment exposure (n=3), that BCMA-TCB2 modulated the transcriptomes of CD4 and CD8 T cells (159 and 141 deregulated genes, respectively), consistent with enhanced activation and T-cell mediated inflammatory response (eg. TNFRS18, STAT1, CCL4). Furthermore, we observed a dose-dependent and significant increment of the CD69 (2-fold), CD25 (2.5-fold) and HLADR (4-fold) activation markers in the surface of NK cells from primary BM samples of 11 MM patients treated with BCMA-TCB2 (Panel E), suggesting a functional crosstalk between activated T cells and NK cells.
In conclusion, we showed that the promising pre-clinical activity of the first-in-class IgG-based BCMA-TCB can be further enhanced by longer treatment periods followed by robust T cell activation. The observation that the efficacy of BCMA-TCB is intrinsically related to the activation status of T cells suggests its rational combination with IMIDs as demonstrated here. Most interestingly, potential crosstalk between activated T and NK cells could lead to enhanced function of the later immune subset, and provide a rational combination between BCMA-TCB and anti-CD38 antibodies to eradicate MM cells through highly activated T and NK cells.
Strein:EngMab: Employment. Vu:EngMab: Employment. Paiva:Celgene: Honoraria, Research Funding; Janssen: Honoraria; Takeda: Honoraria, Research Funding; Sanofi: Consultancy, Research Funding; EngMab: Research Funding; Amgen: Honoraria; Binding Site: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal