To the editor:
The rapid development in treatment options for patients with chronic lymphocytic leukemia (CLL) in parallel with a much more detailed understanding of the underlying pathogenesis has warranted the development of novel prognostic indices for patients with CLL to replace the clinical staging systems developed by Rai and Binet 40 years ago.1,2
Bahlo and colleagues from an international consortium have developed a new international prognostic index for patients with chronic lymphocytic leukemia (CLL-IPI) based on a combination of molecular and clinical baseline characteristics for patients with CLL.3,4 The impact of previously proposed prognostic models has been limited due to omission of molecular characteristics,5 inclusion of parameters not widely used,6 or restriction to cytogenetic findings.7 With an initial assessment of 27 baseline markers in patients enrolled in 8 clinical trials, they have established the CLL-IPI prognostic model based on 5 parameters becoming widely available: TP53 aberrations (including del(17p) and TP53 mutation), IGHV mutational status, β(2)microglobulin level, clinical stage and age. The model was validated in 2 external cohorts including patients followed from time of diagnosis.
The establishment of a robust and widely accepted international prognostic index in CLL to guide treatment decisions and assess the composition of in trial populations is an important and valuable tool.8 The CLL-IPI was developed based on participants in clinical trials before the era of chemoimmunotherapy, with only 571 out of 3725 patients receiving chemoimmunotherapy as first-line treatment. The included patients were younger (median age, 61 years) and mainly physically fit (96% ECOG performance status [PS] 0-1) compared with the general population of newly diagnosed patients with CLL.4 Thus, application and validation of the CLL-IPI in a population-based cohort of patients with newly diagnosed CLL in the current era of chemoimmunotherapy is warranted prior to broader implementation.
Here, we present data from the prospective Danish National CLL Registry, which is a nationwide, mandatory registry including and prospectively following all consecutive patients diagnosed with CLL in Denmark since 2008 to estimate time to event (TTE; treatment or death) and overall survival (OS) according to the 4 CLL-IPI risk groups.9 All prognostic variables were analyzed at the time of diagnosis according to the Danish national guidelines for CLL.
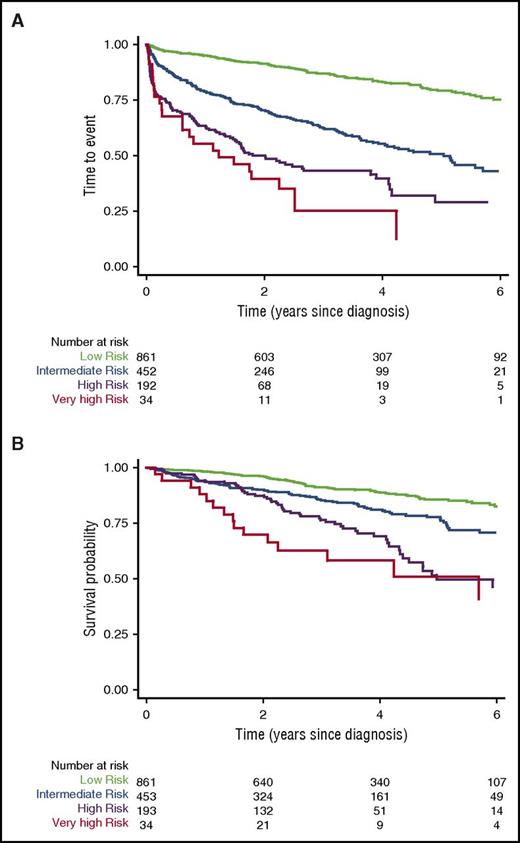
In total, all 5 variables for the CLL-IPI were available for 1514 patients (861 low risk, 453 intermediate risk, 193 high risk, and 34 very high risk) diagnosed with CLL between 2008 and 2015. Excluded from the analyses were an additional 1509 patients included in the registry who were missing 1 or more of the 5 variables. The majority of patients (917 [60%]) were male, the median age was 69 years (interquartile range, 61-76 years), 306 (20%) were Binet stage B or C, 1498 (97%) were PS 0-1, and 3-year OS and 3-year event-free survival rates were 88% and 74%, respectively. 3-year OS in the low-risk, intermediate-risk, high-risk, and very high-risk CLL-IPI groups was 91%, 86%, 76%, and 62%, respectively. A total of 295 patients (19%) (60 low risk [7%], 128 intermediate risk [28%], 87 high risk [45%], and 20 very high risk [59%]) were treated for CLL, and 249 patients (16%) (89 low risk [10%], 89 intermediate risk [20%], 56 high risk [30%], and 15 very high risk [44%]) died during follow-up. The median observation time was 3.2 years, and the median survival was not reached. For patients excluded from the analysis due to ≥1 missing CLL-IPI variables, 898 (61%) were male, 71 years was the median age, 335 (24%) had Binet stage B or C, 1356 (93%) had PS 0-1, the 3-year OS was 80%, and the 3-year event-free survival was 70%.
For our analyses, the 4 different risk categories proposed by Bahlo et al3 predicted significantly different TTE and OS (P < .001) for each of the 4 risk categories (Figure 1). Thus, the robustness of the CLL-IPI index in an unselected cohort of patients with newly diagnosed patients CLL in the era of chemoimmunotherapy could be confirmed.
TTE and OS according to the CLL-IPI. (A) TTE and (B) OS according to the CLL-IPI in 1514 patients with all 5 variables available through the Danish National CLL Registry.
TTE and OS according to the CLL-IPI. (A) TTE and (B) OS according to the CLL-IPI in 1514 patients with all 5 variables available through the Danish National CLL Registry.
As single-agent targeted treatment and combinations of chemotherapy- and non–chemotherapy-based options are evolving, the CLL-IPI may be used to identify at the time of diagnosis CLL patients who will likely not benefit from conventional chemoimmunotherapy, as proposed by Bahlo et al. Our data presented here provide the basis for external validation of the CLL-IPI in a population-based cohort exposed to chemoimmunotherapy. As such, the CLL-IPI could prove a critical step in predicting the time from diagnosis to a need for treatment and help guide therapeutic decision making in the era of novel targeted treatment options for CLL. We encourage all centers caring for patients with CLL to integrate the 5 parameters as part of their routine diagnostic workup and to report the CLL-IPI risk categories for patients in clinical trials.
Authorship
Acknowledgments: The authors thank the Danish hematology centers that participated with data submission to the Danish National CLL Registry. The following physicians contributed to data collection and represent the Danish Hematology centers participating in the Danish National CLL Registry: Christian Hartmann Geisler, Lisbeth Enggaard, Christian Bjørn Poulsen, Peter de Nully Brown, Henrik Frederiksen, Olav Jonas Bergmann, Elisa Jacobsen Pulczynski, Robert Schou Pedersen, and Linda Højberg Nielsen.
Contribution: C.d.C.-B. and I.C. contributed to data analysis and the writing process; and C.U.N. contributed to data collection, study conception, and the writing process.
Conflict-of-interest disclosure: During the study, C.U.N. received grants from the Danish Cancer Society, consultancy fees (from Janssen, Roche, Abbvie, and Gilead), and grants (from Novartis and Roche) outside the submitted work and is the principal investigator for clinical trials sponsored by Roche. The remaining authors declare no competing financial interests.
Correspondence: Carsten Utoft Niemann, Department of Hematology, Rigshospitalet, Copenhagen University Hospital, Building 4042, Blegdamsvej 9, DK-2100 Copenhagen Ø, Denmark; e-mail: carsten.utoft.niemann@regionh.dk.