Key Points
Pevonedistat (MLN4924), a NEDD8-activating enzyme inhibitor, is active in MCL preclinical models and potentiates rituximab activity.
Our findings support further investigation of pevonedistat with or without rituximab in the treatment of MCL.
Abstract
Mantle cell lymphoma (MCL) is characterized by an aggressive clinical course and inevitable development of refractory disease, stressing the need to develop alternative therapeutic strategies. To this end, we evaluated pevonedistat (MLN4924), a novel potent and selective NEDD8-activating enzyme inhibitor in a panel of MCL cell lines, primary MCL tumor cells, and 2 distinct murine models of human MCL. Pevonedistat exposure resulted in a dose-, time-, and caspase-dependent cell death in the majority of the MCL cell lines and primary tumor cells tested. Of interest, in the MCL cell lines with lower half-maximal inhibitory concentration (0.1-0.5 μM), pevonedistat induced G1-phase cell cycle arrest, downregulation of Bcl-xL levels, decreased nuclear factor (NF)-κB activity, and apoptosis. In addition, pevonedistat exhibited additive/synergistic effects when combined with cytarabine, bendamustine, or rituximab. In vivo, as a single agent, pevonedistat prolonged the survival of 2 MCL-bearing mouse models when compared with controls. Pevonedistat in combination with rituximab led to improved survival compared with rituximab or pevonedistat monotherapy. Our data suggest that pevonedistat has significant activity in MCL preclinical models, possibly related to effects on NF-κB activity, Bcl-xL downregulation, and G1 cell cycle arrest. Our findings support further investigation of pevonedistat with or without rituximab in the treatment of MCL.
Introduction
The incorporation of rituximab, high-dose chemotherapy, and autologous stem cell transplant in first remission and/or high-dose cytarabine in the management of mantle cell lymphoma (MCL) has prolonged the median overall survival from between 3 and 4 years to between 5 and 6 years.1-5 Despite dose-intense induction regimens followed by high-dose chemotherapy and autologous stem cell transplant used in the frontline setting, the median time to treatment failure is 4.6 years for all patients and 5.9 years for those younger than 65 years (without a plateau in the curves, with the exception of those patients achieving a molecular remission).1,2 Relapsed/refractory MCL is usually associated with low response rate and/or short duration of response to salvage therapy (including chemotherapy agents or targeted agents such bortezomib or ibrutinib).6,7 There is a need to incorporate promising agents into the treatment of MCL in an attempt to further improve clinical outcomes.
Aberrant expression of Bcl-2 family members in MCL confers resistance to conventional chemotherapy agents.8,9 Deregulation of Bcl-2 family members in B-cell malignancies can be the result of gene translocations, gene amplifications, increased gene transcription, or changes in protein degradation.10 The ubiquitin proteasome system (UPS) is known to regulate Bcl-2 family members indirectly by altering function of the nuclear factor (NF)-κB transcription factor (leading to an increase of Bcl-2, Mcl-1, and Bcl-xL levels) or by degrading proapoptotic Bcl-2–related proteins (Bak).11,12 Proteasome inhibitors like bortezomib have been used in the treatment of lymphoma; however, their use can be limited due to adverse effects. Although the Food and Drug Administration approved bortezomib for the treatment of relapsed/refractory MCL, treatment-related neurotoxicity often precludes dose escalation of this agent and/or its combination with other chemotherapy drugs.
Proteasomal degradation of cellular proteins is a multistep process that requires the “tagging” of targeted proteins with polyubiquitin chains. The ubiquitination of proteins is divided into 3 steps regulated by (1) the ubiquitin-activating enzymes (E1s), (2) the ubiquitin-conjugating enzymes (E2s), and (3) the ubiquitin ligases (E3s). Although E1 and E2 enzymes activate and transfer the ubiquitin, the E3 enzymes selectively recognize the substrates and catalyze the covalent attachment of ubiquitin to the substrates.13
E3s can be further subdivided into HECT (homologous to the E6-AP carboxyl terminus), and RING (really interesting new gene) classes, the latter including finger, U-box, and plant homeodomain finger subtypes. RING finger–type E3s are the largest family and are further subdivided into cullin-based and anaphase-promoting complex ligases, both of which are involved in the proteolysis of core components of the cell cycle. The activation of cullin-based ring-ubiquitin ligases (CRLs) requires neddylation of the cullin subunit, which disrupts its inhibitory binding to the cullin-associated NEDD8-dissociated protein 1.14 Neddylation is a posttranslational modification of the structure, which can alter the function of certain proteins and involves the addition of the ubiquitin-like protein NEDD8 to a target protein (E3). This process is mediated by the NEDD8-activating enzyme (NAE) E1, the NEDD8-conjugating enzyme E2, and the NEDD8-E3 ligase. The diversity of E3 enzymes presents an attractive target for a more selective inhibition of the UPS that can potentially be more clinically effective and less toxic. Pevonedistat (MLN4924) is an investigational NAE inhibitor that selectively prevents the activation of CRLs and alters the ubiquitination and proteasomal degradation of cellular proteins. Inhibition of NAE, and thus CRLs, leads to cell death in cancer models.15-18 In vitro exposure of cancer cell lines to pevonedistat was shown to induce apoptosis, cellular senescence, or autophagy.17-20 In an attempt to evaluate novel therapeutic approaches in MCL, we characterized the preclinical activity of pevonedistat in MCL.
Materials and methods
Cell lines and primary tumor cells
Cytarabine-sensitive or -resistant MCL cell lines were used for the experiments. The Granta, Mino, HBL-2, Z-138, Jeko-1, and Rec-1 cell lines were obtained from the Leibniz-Institute/German Collection of Microorganisms and Cell Cultures (DSMZ). Cytarabine-resistant cell lines (Mino-AraCR, HBL-2-AraCR, Jeko-1-AraCR, and Rec-1-AraCR) were generated and characterized as previously described.21,22 Neoplastic B cells were isolated from pretreatment biopsy tissue obtained from patients with B-cell non-Hodgkin lymphoma or lymphocyte-predominant Hodgkin lymphoma receiving therapy at Roswell Park Cancer Institute (RPCI) as previously described.23
Reagent and antibodies
Pevonedistat (MLN4924) and bortezomib were provided by Millennium Pharmaceuticals (Cambridge, MA). Bendamustine, cytarabine, rituximab (anti-CD20), and trastuzumab (isotype control) were provided by the RPCI pharmacy.
Primary mouse anti-human antibodies against Bak and Bax were obtained from Sigma-Aldrich (St Louis, MO); Bik and Mcl-1 from Santa Cruz Biotechnology (Santa Barbara, CA); poly (ADP-ribose) polymerase 1 (PARP-1) and cleaved PARP-1 from BD Pharmingen (San Jose, CA); and puma from BD Biosciences (San Jose, CA). Primary mouse anti-human antibodies raised against Bcl-2, phospho-Bcl-2, Bcl-xL, p21, cyclin-B, cdc2 (total, Try15, T161), Wee1, Myt1, phospho-H3 (Ser10), CDK7, cyclin-A, Ikkα, phospho-IkBα, and actin were obtained from Cell Signaling Technology (Danvers, MA). Alkaline phosphatase or horseradish peroxidase–conjugated anti-mouse secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA).
Sodium chromate (chromium 51 [51Cr]; PerkinElmer, Boston MA) was used in functional assays assessing antibody-mediated complement cytotoxicity (CMC) or antibody-dependent cellular cytotoxicity (ADCC). Cell Titer-Glo Luminescent Viability Assay reagent was purchased from Promega (Madison, WI). The caspase inhibitor Q-(3S)-5-(2,6-difluorophenoxy)-3-[[(2S)-3-methyl-1-oxo-2-[(2-quinolinylcarbonyl)amino]butyl]amino]-4-oxo-pentanoic acid hydrate (Q-VD-OPh) was purchased from Medical and Biological Laboratories International (Woburn, MA).
In vitro effects of pevonedistat.
Cytarabine-sensitive and -resistant MCL cell lines, as well as primary tumor cells isolated from B-cell lymphoma patients (0.5 × 106 cells per milliliter), were exposed to pevonedistat (0.125-4 μM for cell lines and 0.050-0.5 μM for primary lymphoma samples) or vehicle control (0.001% dimethylsulfoxide) for 24, 48, and 72 hours. Cell viability changes were determined by measuring adenosine triphosphate content using the Cell Titer-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). The half-maximal inhibitory concentration (IC50) of pevonedistat was calculated using the Graph Pad Prism Software Version 6.04 (Graph Pad Software, La Jolla, CA).
Effect of caspase inhibition in pevonedistat antitumor activity.
To assess the contribution of caspase activation on pevonedistat activity, MCL cell lines were incubated with pevonedistat with or without Q-VD-OPh; changes in cell viability were evaluated using the Cell Titer-Glo assay.
In vitro effects of pevonedistat in the cell cycle and induction of apoptosis in MCL.
We selected MCL cell lines with high (Jeko-1 and Rec-1) or low (Granta and HBL-2) IC50 values and evaluated cell cycle and apoptosis threshold changes after NAE inhibition. MCL cell lines were exposed in vitro to pevonedistat (0.25-0.50 μM) for 48 hours. Pevonedistat or vehicle control–exposed cells were harvested, washed with phosphate-buffered saline, and fixed with ice-cold 70% ethanol at −20°C for 30 minutes. Next, cells were incubated with 5 μg/mL of ribonuclease for 30 minutes at room temperature and stained with 5 μg/mL of propidium iodide (PI; Sigma-Aldrich, St Louis, MO) for 1 hour. Differences in the cell cycle distribution or induction of apoptosis (cells in sub-G1 region) after pevonedistat exposure were determined by flow cytometry. Apoptosis induction was confirmed by flow cytometry using annexin-V/PI staining and by western blot analysis (PARP cleavage).
Gene expression profile changes after pevonedistat.
Granta cells were exposed to pevonedistat (250 or 500 nM) or RPMI 1640 in 6-well plates for 24 or 48 hours. The HumanHT-12v4 whole-genome gene expression array was used for expression profiling (Illumina, San Diego, CA). Microarray experiments were performed in triplicate for the conditions tested. The raw intensity of gene expression array was scanned and extracted using BeadScan, with the data corrected by background subtraction in GenomeStudio module. The log2-transformed intensity data were normalized using the quantile normalization function. The limma program was used to calculate the level of gene differential expression for each comparison. Briefly, a linear model was fit to the data (with cell means corresponding to the different conditions and a random effect for array), and a selected contrast for each comparison was performed. The differential expression is set with at least 1.5-fold expression change at P < .05. The list of differentially expressed genes was used for Gene Ontology term enrichment analysis with the National Institutes of Health Database for Annotation, Visualization, and Integrated Discovery Tools.
Changes in Bcl-2 family members or key regulatory proteins of the cell cycle in MCL cell lines after exposure to pevonedistat.
Jeko-1, Rec-1, Granta, and HBL-2 cells were exposed to pevonedistat (0.25-0.50 μM) or vehicle control for 48 hours. Changes in the expression of cell cycle regulatory proteins or Bcl-2 family members were determined by western blot analysis.
In vitro effects of pevonedistat on NF-κB activity in MCL cell lines.
MCL cell lines (2 × 106 cells per milliliter) were exposed to pevonedistat (0.25-1 μM) for 1 or 4 hours. NF-κB activity was determined using a nuclear translocation assay as previously described.24 Changes in Ikkα and phospho-IkBα were determined by western blot analysis.
In vitro effects of pevonedistat with and without chemotherapy on MCL cell lines.
MCL cell lines (0.5 × 106 cells per milliliter) were exposed to pevonedistat (0-1 μM) and/or bendamustine (0-25 μM), cytarabine (0-100 μM), bortezomib (0-15 nM), GS1101 (0.1-10 μM), or ibrutinib (0.1-10 μM) and incubated for 24 to 72 hours. Cell viability was determined using the Cell Titer-Glo assay.
51Cr release assay for the assessment of the impact that pevonedistat exposure has on rituximab-associated CMC and ADCC.
MCL cell lines were exposed to pevonedistat (0.125-1 μM) or dimethylsulfoxide (0.001%) for 48 hours. Subsequently, 2 × 106 viable cells were labeled with 51Cr at 37°C and 5% carbon dioxide for 2 hours. 51Cr-labeled MCL cell lines were plated at a cell concentration of 1 × 105 cells per well (CMC assay) or 1 × 104 cells per well (ADCC assay). Cells were then exposed to rituximab (10 μg/mL) or isotype (10 μg/mL) and to human serum (CMC, 1:4 dilution) or peripheral blood mononuclear cells (ADCC, 40:1 effector: target ratio) for 6 hours at 37°C and 5% carbon dioxide. 51Cr release was measured, and percentage cell lysis was calculated as previously described.25
In vivo effects of pevonedistat alone or in combination with rituximab or systemic chemotherapy in MCL preclinical mouse models.
Two independent sets of experiments were conducted using 2 different strains of severe combined immunodeficient (SCID) mice: (1) natural killer (NK) cell–competent SCID C.B-Igh-1 b/lcrTac-Prkdcscid/Ros (experiments performed at RPCI), and (2) NK cell–deficient nonobese diabetic interleukin-2 receptor γ common-chain null (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) (experiments performed at Charles University).
For in vivo experiments, 6- to 8-week-old mice were used. Animals were bred and maintained at facilities certified by the American Association for Accreditation of Laboratory Animal Care. Both facilities comply with current regulation and standards of the United States Department of Agriculture and the United States Department of Health and Human Services.
In vivo studies used a disseminated human lymphoma–bearing SCID-mouse xenograft model previously described.26 SCID mice were inoculated on day 0 with 10 × 106 Granta cells through tail vein injection. After 24 hours, the animals were divided into 4 cohorts. Each cohort included 15 and 10 mice in the RPCI and Charles University experiments, respectively. The first cohort (group A) was used as control, and the animals did not receive any treatment. Group B consisted of animals treated with rituximab (10 mg/kg) on days 2, 9, 16, and 23 administered via tail vein injection (IV). Group C was treated with pevonedistat (180 mg/kg per dose) given subcutaneously (SQ) on days 1, 4, 8, 11, 15, and 18. Group D was treated with combination treatment of IV rituximab (10 mg/kg) on days 2, 9, 16, and 23 plus SQ pevonedistat (180 mg/kg per dose) on days 1, 4, 8, 11, 15, and 18. The end point of the studies was survival defined as the time to development of limb paralysis.
To evaluate pathological response to pevonedistat in MCL preclinical models, the Clinical Department of Hematology at Charles University removed and analyzed the spleens of several NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice from each of the aforementioned cohorts at the end of therapy (control animals) (day 23) or at the time of the first treated-animal death (day 37). In addition, spleen, liver, and bone marrow specimens were submitted to the department of pathology at RPCI for morphologic assessment of response. Immunohistochemical staining for CD20, Ki67%, and Bcl-1 was used to assess the microscopic response to pevonedistat treatment in MCL-bearing SCID-mouse preclinical models. Morphologic evaluation was performed blinded to the treatment on representative tissue sections from each organ sampled, to evaluate for pleomorphic/blast cytomorphology, tumor burden, necrosis, and mitotic rate (per 10 high power fields, Olympus BX45, ×40 magnification).
To assess the effects of pevonedistat in combination with chemotherapy, 6- to 8-week-old SCID mice (C.B-Igh-1 b/lcrTac-Prkdcscid/Ros) were inoculated on day 0 with 10 × 106 Granta cells through tail vein injection. After 72 hours, the animals were divided into 4 cohorts. Each cohort included 15 animals. Group A was used as control, and the animals did not receive any treatment. Group B consisted of animals treated with cytarabine administered intraperitoneally (1000 mg/kg per dose) on days 5, 12, 19, and 26 . Group C was treated with SQ pevonedistat (180 mg/kg per dose) on days 3, 6, 10, 13, 17, and 20. Group D was treated with a combination treatment of intraperitoneal cytarabine (1000 mg/kg per dose) on days 5, 12, 19, and 26 plus SQ pevonedistat (180 mg/kg per dose) on days 3, 6, 10, 13, 17, and 20. The end point of the studies was survival defined as the time to development of limb paralysis.
Statistics
All experiments were performed in triplicate on 3 separate occasions. Data were plotted and analyzed using SPSS 21.0 software. For in vitro and ex vivo studies, statistical differences between treatment groups and controls were determined by Student t test. For in vitro studies combining pevonedistat and chemotherapy drugs, the coefficient of synergy was calculated using the Calcusyn Software Version 2.11 (Biosoft, Great Shelford, Cambridge, United Kingdom). In addition, differences in survival between treatment groups were calculated using Kaplan-Meier curves. A P value of <.05 was defined as having statistical significance.
Results
Pevonedistat induces cell death in MCL cell lines and in primary tumor cells
Exposure to pevonedistat led to a time- and dose- dependent decrease in viability for all cell lines evaluated. IC50 levels varied between 78 nM for the most sensitive cell line (Granta) and 8445 nM for the most resistant one (Rec-1) (Figure 1A-B). Activity was observed in cytarabine-sensitive or -resistant cell lines. A variable degree of cell death was observed in primary tumor cells isolated from B-cell lymphoma patients, including MCL (Figure 1C-D).
Pevonedistat induces a decrease in the cell viability of MCL cell lines or primary tumor cells isolated from lymphoma patients. (A) In vitro exposure of MCL cell lines to pevonedistat resulted in dose- and time-dependent (48-hour time point shown) cell death in cytarabine-sensitive cells (Granta, HBL-2, Mino, Z-138, Jeko-1, Rec-1) and cytarabine-resistant cells (HBL-2 AraCR, Mino AraCR, Jeko-1 AraCR, Rec-1 AraCR). Cytarabine-sensitive and -resistant cells were exposed to escalating doses of pevonedistat (0-4000 nM) for 24, 48, and 72 hours. Cell death was determined by Cell Titer-Glo luminescence assay. (B) Pevonedistat IC50 levels at 48 hours. (C) Ex vivo exposure of primary lymphoma cells derived from patients with MCL to pevonedistat resulted in varying degrees of cell death. Pevonedistat or vehicle control was used at 500 nM in MCL cells. Cell death was determined by Cell Titer-Glo luminescence assay, to detect adenosine triphosphate generation after 48 hours. All experiments were repeated 3 separate times and were reported as the median with standard deviation error bars. (D) Clinical and pathological characteristics of the patient from which primary tumor cells were obtained. *P < .05.
Pevonedistat induces a decrease in the cell viability of MCL cell lines or primary tumor cells isolated from lymphoma patients. (A) In vitro exposure of MCL cell lines to pevonedistat resulted in dose- and time-dependent (48-hour time point shown) cell death in cytarabine-sensitive cells (Granta, HBL-2, Mino, Z-138, Jeko-1, Rec-1) and cytarabine-resistant cells (HBL-2 AraCR, Mino AraCR, Jeko-1 AraCR, Rec-1 AraCR). Cytarabine-sensitive and -resistant cells were exposed to escalating doses of pevonedistat (0-4000 nM) for 24, 48, and 72 hours. Cell death was determined by Cell Titer-Glo luminescence assay. (B) Pevonedistat IC50 levels at 48 hours. (C) Ex vivo exposure of primary lymphoma cells derived from patients with MCL to pevonedistat resulted in varying degrees of cell death. Pevonedistat or vehicle control was used at 500 nM in MCL cells. Cell death was determined by Cell Titer-Glo luminescence assay, to detect adenosine triphosphate generation after 48 hours. All experiments were repeated 3 separate times and were reported as the median with standard deviation error bars. (D) Clinical and pathological characteristics of the patient from which primary tumor cells were obtained. *P < .05.
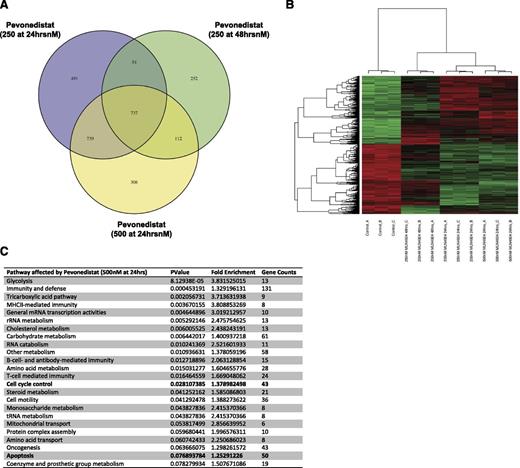
Gene expression profile changes after in vitro exposure to pevonedistat
To define the activation of key regulatory pathways after pevonedistat therapy, we performed gene expression profiling studies in Granta cells. Using the Illumina HumanHT-12v4 whole-genome bead chip, we tested changes in gene expression profiles after pevonedistat drug exposure for 24 or 48 hours. As expected, in vitro exposure to pevonedistat resulted in significant gene expression changes observed in all cell lines tested, and we identified a total of 737 genes consistently upregulated or downregulated after pevonedistat exposure (Figure 2A-B). The complete data are available in supplemental Tables 1 and 2, available on the Blood Web site. Gene pathways affected included the cell cycle and apoptosis pathways (Figure 2C). Changes in protein expression were also confirmed by western blot analysis (Figures 3A and 4B).
Gene expression changes of key regulatory pathways in MCL (Granta cells) exposed to pevonedistat. (A) Granta cells were exposed to pevonedistat for 24 hours (250 and 500 nM) or 48 hours (250 nM) and then placed on Illumina HumanHT-12v4 whole-genome expression microarray. A total of 737 genes were found to be differentially expressed in all conditions tested. (B) Hierarchical clustering of expression profiles of the 737 genes differentially expressed in all the cell line exposed to pevonedistat. In the clustering heat map, red indicates upregulation, whereas green indicates downregulation. (C) List of the cellular pathways of genes affected by pevonedistat exposure (500 nM for 24 hours shown).
Gene expression changes of key regulatory pathways in MCL (Granta cells) exposed to pevonedistat. (A) Granta cells were exposed to pevonedistat for 24 hours (250 and 500 nM) or 48 hours (250 nM) and then placed on Illumina HumanHT-12v4 whole-genome expression microarray. A total of 737 genes were found to be differentially expressed in all conditions tested. (B) Hierarchical clustering of expression profiles of the 737 genes differentially expressed in all the cell line exposed to pevonedistat. In the clustering heat map, red indicates upregulation, whereas green indicates downregulation. (C) List of the cellular pathways of genes affected by pevonedistat exposure (500 nM for 24 hours shown).
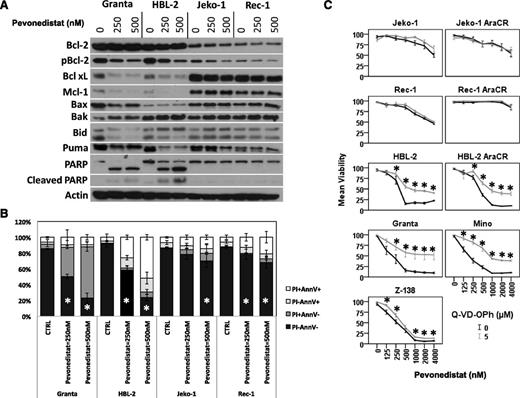
Pevonedistat induces apoptosis in MCL cell lines. (A) In vitro exposure of MCL cell lines to pevonedistat for 48 hours altered the balance of proapoptotic (Bak) and antiapoptotic (Bcl-xL and Mcl-1) Bcl-2 family members, leading to PARP cleavage. (B) Induction of apoptosis after pevonedistat was further tested by flow cytometry studies using annexin-V (AnnV)/PI staining at 48 hours. (C) Inhibition of caspase activation partially rescued MCL cell lines to the apoptotic effects of pevonedistat. Cytarabine-sensitive and -resistant cells were exposed in vitro to pevonedistat (0-4000 nM) with or without a pan-caspase inhibitor (Q-VD-OPh, 5 μM); cell viability was determined with Cell Titer-Glo luminescent assay after 48 hours. CTRL, control. *P < .05.
Pevonedistat induces apoptosis in MCL cell lines. (A) In vitro exposure of MCL cell lines to pevonedistat for 48 hours altered the balance of proapoptotic (Bak) and antiapoptotic (Bcl-xL and Mcl-1) Bcl-2 family members, leading to PARP cleavage. (B) Induction of apoptosis after pevonedistat was further tested by flow cytometry studies using annexin-V (AnnV)/PI staining at 48 hours. (C) Inhibition of caspase activation partially rescued MCL cell lines to the apoptotic effects of pevonedistat. Cytarabine-sensitive and -resistant cells were exposed in vitro to pevonedistat (0-4000 nM) with or without a pan-caspase inhibitor (Q-VD-OPh, 5 μM); cell viability was determined with Cell Titer-Glo luminescent assay after 48 hours. CTRL, control. *P < .05.
Pevonedistat exposure results in variable degrees of cell cycle arrest that correlates with changes in key regulatory proteins of the cell cycle progression (p21 and CDC2). (A) MCL cell lines were exposed to pevonedistat (250 or 500 nM) for 48 hours. Subsequently, cells were fixed, stained with PI, and analyzed by flow cytometry. (B) MCL cell lines were exposed to pevonedistat (250 or 500 nM) or vehicle control. After 48 hours of drug exposure, changes in cell cycle regulatory proteins were studied by western blot analysis.
Pevonedistat exposure results in variable degrees of cell cycle arrest that correlates with changes in key regulatory proteins of the cell cycle progression (p21 and CDC2). (A) MCL cell lines were exposed to pevonedistat (250 or 500 nM) for 48 hours. Subsequently, cells were fixed, stained with PI, and analyzed by flow cytometry. (B) MCL cell lines were exposed to pevonedistat (250 or 500 nM) or vehicle control. After 48 hours of drug exposure, changes in cell cycle regulatory proteins were studied by western blot analysis.
In vitro exposure of pevonedistat alters the balance of several Bcl-2 family member proteins favoring the induction of apoptosis
In cell lines with low IC50 values (Granta and HBL-2), pevonedistat induced a dose-dependent decrease in the expression of the antiapoptotic Bcl-2 family member proteins Bcl-xL, Mcl-1, and pBcl-2 (Figure 3A). In addition, pevonedistat induced a dose-dependent decrease in proapoptotic cleaved Bid and an upregulation of Bak (Figure 3A). Perhaps related to these changes, PARP cleavage was observed, suggesting that pevonedistat induced cell apoptosis in sensitive MCL cell lines (Figure 3). However, MCL with higher pevonedistat IC50 values exhibited no changes in the expression of Bcl-2 family members at the doses tested (Figure 3A).
Pevonedistat induces apoptosis in a dose-dependent manner in MCL cell lines
We used annexin V/PI staining to further confirm whether exposure to pevonedistat induced apoptosis in MCL cell lines. In sensitive cells, pevonedistat induced apoptosis in a dose-dependent manner (Figure 3B).
Caspase inhibition partially rescues MCL cell lines from the cytotoxic effects of pevonedistat
Preincubation of MCL cell lines with Q-VD-OPh increased the cell viability after pevonedistat drug exposure (Figure 3C). In MCL cell lines with higher IC50 values, caspase inhibition had no effect on pevonedistat cytotoxic effects at higher doses, suggesting that pevonedistat can reduce cell viability by both caspase-dependent and -independent mechanisms.
Pevonedistat induces G1 or S cell cycle arrest that was associated with specific changes in cell cycle regulatory proteins in MCL cell lines
To further study caspase-independent mechanisms of cell death in pevonedistat-exposed cells, we studied the effect of NAE inhibition in cell cycle regulatory proteins and cell cycle progression. Of interest, only MCL cell lines with low pevonedistat IC50 values (Granta and HBL-2) underwent G1 cell cycle arrest upon NAE inhibition (Figure 4A). Granta cells exposed to pevonedistat also exhibited an accumulation of cells in S phase. In vitro exposure to pevonedistat led to the dose-dependent stabilization of p21 and, to a lesser degree, Wee1 in all cell lines tested (Figure 4B). In addition, downregulation of cyclin B1, cdc2, pCdc2 (T15), pCdc2 (T161), Myt1, and CDK7 was observed only in the Granta and HBL-2 cell lines (Figure 4B).
Pevonedistat decreases NF-κB activity in MCL cell lines
NF-κB activity is known to be tightly regulated by the UPS, and translocation of p65 into the nuclei leads to the increased transcription of antiapoptotic Bcl-2 family member genes. Similarly to what has been observed with other inhibitors of the UPS (ie, bortezomib or carfilzomib), pevonedistat decreased NF-κB activity as demonstrated by p65 colocalization studies (Figure 5A). Perhaps related to this, pevonedistat exposure led to the accumulation of phosphorylated IkBα by preventing its degradation by the UPS and the decrease in protein expression of IKKα (Figure 5B).
In vitro exposure of MCL cell lines to pevonedistat decreases NF-κB activity. (A) MCL cell lines were exposed to pevonedistat (125-250 nM) for up to 4 hours. Changes in NF-κB activation were analyzed using ImageStream technology. Nuclear p65 translocation into the nuclei is reported as similarity score (SS) of Dapi and p65 images. The RD metric reports the changes in SS by measuring the shift between 2 distributions. (B) Changes in the protein stability of Ikkα and phospho (P)-IkBα were analyzed through the use of western blotting after pevonedistat in vitro exposure. PMA/Ion, phorbol 12-myristate 13-acetate/ionomycin.
In vitro exposure of MCL cell lines to pevonedistat decreases NF-κB activity. (A) MCL cell lines were exposed to pevonedistat (125-250 nM) for up to 4 hours. Changes in NF-κB activation were analyzed using ImageStream technology. Nuclear p65 translocation into the nuclei is reported as similarity score (SS) of Dapi and p65 images. The RD metric reports the changes in SS by measuring the shift between 2 distributions. (B) Changes in the protein stability of Ikkα and phospho (P)-IkBα were analyzed through the use of western blotting after pevonedistat in vitro exposure. PMA/Ion, phorbol 12-myristate 13-acetate/ionomycin.
Pevonedistat affects the cytotoxic activity of chemotherapy agents and small-molecule inhibitors
Nawrocki et al demonstrated that pevonedistat has synergistic effects with chemotherapy agents in acute myelogenous leukemia (AML) models (ie, cytarabine).27 Similarly, in MCL cell lines, pevonedistat exhibited synergistic/additive effects when combined with cytarabine, bendamustine, bortezomib, GS1101, or ibrutinib (Figure 6A-D,F). Pevonedistat in combination with cytarabine improved the median survival (78 days) compared with cytarabine (25 days) or pevonedistat monotherapy (53 days) (P = .002 and P = .002) (Figure 6E).
In vitro and in vivo effects of pevonedistat on the antitumor activity of chemotherapy agents and B-cell receptor signaling inhibitors. In vitro exposure of a panel of MCL cell lines to pevonedistat potentiated the cytotoxic effects of bortezomib (A), bendamustine (B), or cytarabine (C). (D) Coefficient of synergy demonstrated that the combination of pevonedistat and cytarabine resulted in synergistic activity (green) in several MCL cell lines. (E) Pevonedistat exhibits synergistic activity in combination with cytarabine in a SCID-mouse model of MCL (C.B-Igh-1 b/lcrTac-Prkdcscid/Ros). The combination of pevonedistat (180 mg/kg per dose) and cytarabine (1000 mg/kg per dose) was more effective in controlling lymphoma growth and prolonging survival than pevonedistat or cytarabine as a single agent. Survival differences between groups were compared using log rank analysis. (F) In addition, pevonedistat exhibited synergistic activity when combined with ibrutinib or GS1101.
In vitro and in vivo effects of pevonedistat on the antitumor activity of chemotherapy agents and B-cell receptor signaling inhibitors. In vitro exposure of a panel of MCL cell lines to pevonedistat potentiated the cytotoxic effects of bortezomib (A), bendamustine (B), or cytarabine (C). (D) Coefficient of synergy demonstrated that the combination of pevonedistat and cytarabine resulted in synergistic activity (green) in several MCL cell lines. (E) Pevonedistat exhibits synergistic activity in combination with cytarabine in a SCID-mouse model of MCL (C.B-Igh-1 b/lcrTac-Prkdcscid/Ros). The combination of pevonedistat (180 mg/kg per dose) and cytarabine (1000 mg/kg per dose) was more effective in controlling lymphoma growth and prolonging survival than pevonedistat or cytarabine as a single agent. Survival differences between groups were compared using log rank analysis. (F) In addition, pevonedistat exhibited synergistic activity when combined with ibrutinib or GS1101.
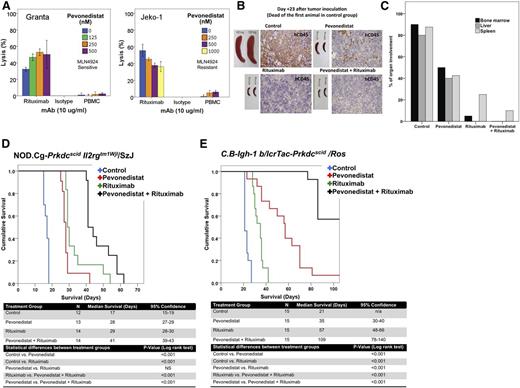
Pevonedistat enhances rituximab activity in vitro and in vivo
Previous investigators have demonstrated that ADCC is an important mechanism of action of rituximab in vitro and in vivo.26,28 Preincubation of MCL cell lines with pevonedistat in vitro for up to 48 hours prior to rituximab exposure enhanced rituximab-mediated ADCC (Figure 7A) but not CMC (data not shown). More important, pevonedistat enhanced the antitumor activity of rituximab in vivo in 2 different MCL mouse models (Figure 7B-E).
Pevonedistat potentiates the antitumor activity of rituximab in vitro and in vivo. (A) Preincubation of MCL cell lines for 48 hours with pevonedistat increased rituximab-associated ADCC in vitro. Both the Granta cell line (pevonedistat-sensitive) and the Jeko-1 cell line (pevonedistat-resistant) were evaluated. ADCC was assessed using standard 51Cr release assays. Each experiment was done in triplicate. (B-E) Pevonedistat exhibits synergistic activity in combination with rituximab in 2 SCID-mouse models: NK cell–deficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (B-D) and NK cell–competent C.B-Igh-1 b/lcrTac-Prkdcscid/Ros (E). (B) Gross and microscopic response to pevonedistat exposure in MCL preclinical in vivo models were also evaluated via necropsy and investigation of mouse spleens from each treatment group (control, rituximab, pevonedistat, or pevonedistat plus rituximab), as well as assessment of human (h)CD45 by immunohistochemistry. (C) In vivo treatment of NK cell–deficient SCID mice bearing the Granta cell lines with pevonedistat, rituximab, or the combination of both agents resulted in a decreased tumor burden when compared with control animals. The combination of pevonedistat and rituximab resulted in a larger decrease of lymphoma involvement in several organs as determined by pathological analysis. (D-E) In vivo treatment of MCL-bearing SCID mice with rituximab and pevonedistat resulted in synergistic activity when compared with single agent–treated or control-treated animals. Survival differences between groups were compared using log rank analysis. Experiments were repeated 3 separate times. MAb, monoclonal antibody; n/a, not applicable; NS, not significant; PBMC, peripheral blood mononuclear cell.
Pevonedistat potentiates the antitumor activity of rituximab in vitro and in vivo. (A) Preincubation of MCL cell lines for 48 hours with pevonedistat increased rituximab-associated ADCC in vitro. Both the Granta cell line (pevonedistat-sensitive) and the Jeko-1 cell line (pevonedistat-resistant) were evaluated. ADCC was assessed using standard 51Cr release assays. Each experiment was done in triplicate. (B-E) Pevonedistat exhibits synergistic activity in combination with rituximab in 2 SCID-mouse models: NK cell–deficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (B-D) and NK cell–competent C.B-Igh-1 b/lcrTac-Prkdcscid/Ros (E). (B) Gross and microscopic response to pevonedistat exposure in MCL preclinical in vivo models were also evaluated via necropsy and investigation of mouse spleens from each treatment group (control, rituximab, pevonedistat, or pevonedistat plus rituximab), as well as assessment of human (h)CD45 by immunohistochemistry. (C) In vivo treatment of NK cell–deficient SCID mice bearing the Granta cell lines with pevonedistat, rituximab, or the combination of both agents resulted in a decreased tumor burden when compared with control animals. The combination of pevonedistat and rituximab resulted in a larger decrease of lymphoma involvement in several organs as determined by pathological analysis. (D-E) In vivo treatment of MCL-bearing SCID mice with rituximab and pevonedistat resulted in synergistic activity when compared with single agent–treated or control-treated animals. Survival differences between groups were compared using log rank analysis. Experiments were repeated 3 separate times. MAb, monoclonal antibody; n/a, not applicable; NS, not significant; PBMC, peripheral blood mononuclear cell.
We investigated the effect of pevonedistat treatment in the NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ strain MCL preclinical mouse model, by observing both the gross and microscopic responses to treatment with pevonedistat, rituximab, or pevonedistat in combination with rituximab. Macroscopic and microscopic pathological examination of organs obtained at different time intervals demonstrated that pevonedistat in combination with rituximab resulted in the best pathological response (Figure 7B-C). In this particular mouse model known to lack NK cells, rituximab or pevonedistat as a single agent exhibited modest antitumor activity. Of interest, the combination of rituximab and pevonedistat resulted not only in better disease control as demonstrated by histopathological studies but also in improved survival when compared with rituximab or pevonedistat monotherapy (Figure 7D).
In an NK cell–competent mouse model (RPCI model, strain C.B-Igh-1 b/lcrTac-Prkdcscid/Ros), pevonedistat monotherapy prolonged the survival of MCL-bearing SCID mice when compared with controls (median, 57 vs 21 days; P < .05). The median survival was 109 days in animals treated with pevonedistat in combination with rituximab, whereas the median survival for animals receiving rituximab monotherapy was only 57 days (P < .001) (Figure 7E). In both MCL-bearing SCID-mouse models, pevonedistat in combination with rituximab resulted in synergistic activity (Figure 7E).
Discussion
Preclinical studies and clinical trials have demonstrated that bortezomib or carfilzomib enhance the antitumor activity of chemotherapy agents and/or rituximab in lymphoid malignancies.29-32 However, treatment-related toxicities have limited the dose escalation of these agents in the clinical setting. We are interested in targeting the NAE, a component of the UPS, as an alternative to targeting the proteasome in B-cell lymphoma, hypothesizing that it may be associated with a better therapeutic index than proteasome inhibitors.
Pevonedistat had been shown to be active in activated B-cell/diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and AML preclinical models, and exhibits synergistic activity when combined with B-cell receptor inhibitors or cytarabine.20,27,33 Pharmacokinetics/efficacy modeling conducted at Millennium Pharmaceuticals has demonstrated that efficacy of MLN4924 (pevonedistat) is related to the total exposure during the treatment period. Although the dose of 180 mg/kg was higher in our studies in MCL models than in papers reporting dosing of 30 mg/kg or 60 mg/kg, in our MCL studies, it was administered only twice weekly for a total weekly dose of 360 mg/kg per week, whereas the papers using lower doses reported administering the compound twice daily every day, for a similar or even higher total weekly dose (420-840 mg/kg per week).34 The intermittent dosing schedule used in the MCL models has also been used in studies of AML models.
Using MCL preclinical models, we found that pevonedistat reduced NF-κB activity and decreased Bcl-xL expression, resulting in apoptosis. In contrast to what was observed by other investigators, we did not see any changes in Bim expression or consistent Bid cleavage. Although Bid cleavage is recognized as an important component of the general apoptotic response, it does not appear to be absolutely required for apoptosis in lymphoid cells. Kaufmann et al reported that loss of Bid in mouse B cells provided no protection against chemotherapy or γ-radiation exposure.35 Based on the increase in PARP cleavage in Figure 2A, we believe that the decrease in expression of Bcl-xL and Mcl-1 in the Granta and HBL-2 cell lines after exposure to pevonedistat is enough to make up for the decrease in Bid and Bid expression. Likely related to changes in Bcl-2 family members, pevonedistat enhanced the antitumor activity of chemotherapy agents and/or rituximab in our MCL preclinical models.
The mechanisms by which pevonedistat potentiates the rituximab-associated ADCC in vitro or in vivo are not yet determined. It is possible that tilting the balance toward apoptosis can result by way of the downregulation of Bcl-xL after pevonedistat drug exposure and that the interactions of granzyme B released during rituximab-associated ADCC and/or the calcium influx after CD20 binding leading to Bim release from the microtubules may play role in this phenomenon.
Pevonedistat is undergoing clinical evaluation in patients with refractory malignancies. A phase 1 study evaluating the maximum tolerated dose and safety of pevonedistat in patients with relapsed/refractory lymphoma or multiple myeloma has been completed.36 Enrolled patients were scheduled to receive pevonedistat (at different dose levels) as a 60-minute IV infusion on days 1, 2, 8, and 9 (schedule A); on days 1, 4, 8, and 11 (schedule B); or on days 1 and 8 (schedule C) of 21-day cycles for up to 12 months. A report was issued after the first 7 patients were treated with pevonedistat. At the time of the report, no dose-limiting toxicity was observed. Common side effects included fatigue, nausea, myalgia, and elevated liver enzymes.36
Pevonedistat has been evaluated in patients with relapsed/refractory AML or myelodysplastic syndrome.37 Two dose schedules were tested among 53 patients: pevonedistat was administered at escalating doses on days 1, 3, and 5 every 21 days (schedule A) or on days 1, 4, 8, and 11 every 21 days (schedule B). Dose-limiting toxicities included grade 3 transaminase elevation, grade 3 thrombocytopenia, orthostatic hypotension, and cardiac failure. The overall complete and partial response rates in patients treated at or below the maximum tolerated dose were 17% (4/23; 2 complete and 2 partial responses) for schedule A and 10% (2/19; 2 partial responses) for schedule B.37 Schedule A was selected for further clinical development, and a phase 1b investigation of pevonedistat combined with azaciditine is ongoing in patients with AML (NCT01814826).
In summary, the multiple cellular events observed after pevonedistat exposure suggest that targeting NAE affects key regulatory proteins of the apoptotic machinery and/or cell cycle. Likely related to this, pevonedistat exhibited synergistic activity with rituximab, cytarabine, bendamustine, and B-cell receptor signaling inhibitors (ibrutinib or GS1101). Our preclinical data support the further evaluation of pevonedistat alone or in combination with rituximab and with or without systemic chemotherapy in clinical trials in patients with relapsed/refractory MCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health National Cancer Institute (5RO1CA136907-02), The Eugene and Connie Corasanti Lymphoma Research Fund, the Czech Ministry of Health (IGA-MZ NT13201-4/2012), and by institutional research grants PRVOUK-27/LF1/1 and PRVOUK P24/LF1/3.
Authorship
Contribution: N.M.C., M.J.B., J.G., C.M., Q.H., V.N., P.K., P.V., and S.E.F. performed the research; F.J.H.-I., S.L., P.K., and M.S.C. designed the research study; V.N. evaluated key pathological material; N.M.C., J.G., M.J.B., C.M., P.K., and F.J.H.-I. analyzed the data; and N.M.C., M.S.C., and F.J.H.-I. wrote the paper.
Conflict-of-interest disclosure: M.S.C. has served on an advisory board for Millennium Pharmaceuticals. F.J.H.-I. has served on an advisory board for Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Francisco J. Hernandez-Ilizaliturri, Departments of Medicine and Immunology, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263; e-mail: francisco.hernandez@roswellpark.org.