Key Points
Ibrutinib treatment of CLL enhances the generation of CAR T cells for adoptive immunotherapy.
Concurrent ibrutinib therapy improves the engraftment and therapeutic efficacy of anti-CD19 CAR T cells in mouse models.
Abstract
Anti-CD19 chimeric antigen receptor (CAR) T-cell therapy is highly promising but requires robust T-cell expansion and engraftment. A T-cell defect in chronic lymphocytic leukemia (CLL) due to disease and/or therapy impairs ex vivo expansion and response to CAR T cells. To evaluate the effect of ibrutinib treatment on the T-cell compartment in CLL as it relates to CAR T-cell generation, we examined the phenotype and function of T cells in a cohort of CLL patients during their course of treatment with ibrutinib. We found that ≥5 cycles of ibrutinib therapy improved the expansion of CD19-directed CAR T cells (CTL019), in association with decreased expression of the immunosuppressive molecule programmed cell death 1 on T cells and of CD200 on B-CLL cells. In support of these findings, we observed that 3 CLL patients who had been treated with ibrutinib for ≥1 year at the time of T-cell collection had improved ex vivo and in vivo CTL019 expansion, which correlated positively together and with clinical response. Lastly, we show that ibrutinib exposure does not impair CAR T-cell function in vitro but does improve CAR T-cell engraftment, tumor clearance, and survival in human xenograft models of resistant acute lymphocytic leukemia and CLL when administered concurrently. Our collective findings indicate that ibrutinib enhances CAR T-cell function and suggest that clinical trials with combination therapy are warranted. Our studies demonstrate that improved T-cell function may also contribute to the efficacy of ibrutinib in CLL. These trials were registered at www.clinicaltrials.gov as #NCT01747486, #NCT01105247, and #NCT01217749.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent adult leukemia and is characterized by a progressive accumulation of incompetent B lymphocytes that are monoclonal in origin. A central driving feature of CLL pathogenesis is early immune deficiency, which promotes tumor expansion and evasion of immune surveillance.1,2 Studies of innate and adaptive immune system function in CLL show that absolute numbers of natural killer cells and T cells, as well as hypogammaglobulinemia at diagnosis, are predictive of overall survival.3-6 T-cell immune suppression in CLL may be mediated by microenvironment-driven immune suppression and the expression of T-cell inhibitory checkpoint ligands and their receptors such as programmed death ligand 1 (PD-L1) and programmed cell death 1 (PD-1); several commonly used treatments (eg, fludarabine and alemtuzumab) further compound immunosuppression by profoundly depleting T cells. Although allogeneic stem cell transplant can be curative, even reduced-intensity treatment regimens have significant morbidity and mortality in the CLL population due to comorbidities and acute/chronic graft-versus-host disease.
Recent studies have demonstrated that durable remissions are possible in relapsed and refractory CLL and acute lymphocytic leukemia (ALL) patients infused with autologous T cells genetically modified with a chimeric antigen receptor (CAR) directed to CD19.7-10 CTL019 is a second-generation anti-CD19 CAR introduced into T cells with a lentiviral vector as part of an ex vivo manufacturing process. The manufacturing process itself requires T-cell proliferation, and because T cells from CLL patients are difficult to expand, we routinely perform a small-scale test expansion before embarking on large-scale manufacturing.11 The efficacy of CTL019 is associated with a strong proliferative response in vivo, as well as persistence of the gene-modified T cells.11 In cases of relapse after robust and persistent T-cell expansion for ALL and CLL, tumor silencing or modification of the CD19 antigen is often noted, thus directly implicating the CTL019-CD19 interaction in mediating an antitumor response and underscoring the strong selective pressure that the presence of CTL019 cells have on CD19-expressing cells.12,13 Studies with CTL019 have shown that the complete response (CR) rates in relapsed or refractory CLL are much lower than in relapsed or refractory ALL patients (∼20%-25% vs 90%); other groups have also noted poor efficacy of different types of CAR T cells in CLL compared with ALL.11,14-16 Thus, intrinsic T-cell defects in CLL impose a significant barrier to both the feasibility of generating CAR T cells and the responsiveness of the disease to CAR T cell–based therapy.
We hypothesized that the state of the endogenous T-cell compartment contributes to the feasibility and efficacy of CAR T-cell therapy in hematologic malignancies, and that T cells from patients with CLL have a poor functional capacity due to disease, treatment, or both. Many standard therapies for CLL, including alkylators, fludarabine, bendamustine, corticosteroids, and alemtuzumab, have a profound negative impact on T-cell function, which likely exacerbates the T-cell defect in CLL. However, ibrutinib, the first-in-class irreversible inhibitor of Bruton tyrosine kinase (BTK), may not only avoid negative effects on the T-cell compartment but could also potentially improve antitumor T-cell immunity. For example, ibrutinib inhibits the interleukin (IL)-2 inducible T-cell kinase (ITK) in immunosuppressive T helper (Th)2-type CD4+ T cells, with enhancement in immune function toward several Th1-driven infections.17 In addition, another report demonstrated that combining ibrutinib with PD-1 blockade can improve T-cell responses against solid tumors that do not express BTK.18 These observations led us to further hypothesize that ibrutinib could enhance CAR T-cell therapy, either during the cell expansion/manufacturing stage or when administered concurrently with CAR T cells. Our results show that prolonged treatment with ibrutinib restored CLL patient T-cell functions as measured ex vivo and that concurrent ibrutinib treatment enhanced CAR T-cell activity and engraftment in xenograft models of leukemia.
Methods
Subject populations
Blood samples were obtained from patients enrolled in institutional review board–approved clinical trials of CTL019 or ibrutinib, and written informed consent was provided in accordance with the Declaration of Helsinki. For additional information, see supplemental Methods, available on the Blood Web site.
Cell lines, DNA constructs, and generation of CTL019 cells
The OSU-CLL cell line was established and maintained in culture as previously described.19 Wild-type parental K562 cells (purchased from American Type Culture Collection), K562 cells engineered to express CD19 (K562-CD19) along with PD-L1 (K562-CD19-PDL1), and Nalm-6 cells (American Type Culture Collection) were used as targets where indicated in the relevant experiments. Primary donor T cells were transduced with the anti-CD19 CAR containing a 4-1BB intracellular signaling domain (CTL019) and expanded as previously reported.20 Details of primary T-cell activation, lentiviral transduction, and messenger (m)RNA transfection are provided in supplemental Methods.
Molecular analyses
In CTL019 patients, total genomic DNA was isolated from cryopreserved peripheral blood mononuclear cells at defined pre- or postinfusion time points. Quantitative polymerase chain reaction analysis of genomic DNA samples was performed in bulk using 200 ng of genomic DNA per condition, Applied Biosystems TaqMan technology, and a validated assay to detect the integrated anti-CD19 CAR transgene sequence.8 Each data point (samples, standard curve, reference controls) was derived from triplicate measurements, with average values reported.
In vitro functional studies
Small-scale ex vivo expansions from peripheral blood samples and clinical-grade expansions of leukapheresis products were performed in the Clinical Cell and Vaccine Production Facility as described.7 Carboxyfluorescein succinimidyl ester–labeled T cells21 were incubated with media alone or increasing concentrations of ibrutinib at the specified concentrations. Anti-CD3/CD28 Dynabeads (ThermoFisher Scientific) were subsequently added to treated or untreated T-cell cultures, and proliferation was assessed by monitoring carboxyfluorescein succinimidyl ester dilution. Absolute cell counts during ex vivo expansions of unmodified or anti-CD19 CAR transgene–modified T cells were obtained using a Coulter Counter (Beckman Coulter). Population doublings were calculated using the equation At = A02n, where n is the number of population doublings, A0 is the input number of cells, and At is the total number of cells. For intracellular cytokine analysis, T cells were left unstimulated or incubated with anti-CD3/CD28 beads or phorbol myristate acetate/ionomycin for 6 hours in the presence of monensin.
Flow cytometry
Cells were stained with antibodies against immunophenotypic markers and analyzed on an LSRFortessa (BD Biosciences). Additional information is provided in supplemental Methods.
Animal experiments
All animal procedures were performed in accordance with Federal and Institutional Animal Care and Use Committee requirements. Detailed methods and end points for the mouse models and the studies performed are provided in supplemental Methods.
Statistical analysis
Unless otherwise stated, a 2-tailed Student t test was used for normal data at equal variance. Survival curves were generated using the Kaplan-Meier method, and statistical comparisons were performed using the log-rank (Mantel-Cox) test. Significance was considered for P < .05. For the detailed statistics analysis plan, see supplemental Methods.
Results
T cells in CLL disease exhibit a proliferative defect
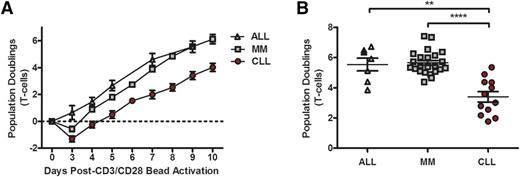
One of the most stringent measures of T-cell function is the proliferative burst that can be achieved via a single stimulation with artificial antigen-presenting cells; importantly, this proliferative burst is also essential in generating CAR T cells. We found that T cells derived from CLL patients exhibited reduced proliferative capacity compared with similarly aged patients with relapsed or refractory multiple myeloma or young adults with ALL (Figure 1A). The proliferative burst is apparent in the first 3 days of culture and resulted in a significantly lower yield of T cells from patients with CLL at the end of expansion (Figure 1B). In this CD3/CD28 bead–based expansion protocol, the CD4/CD8 ratio of the starting cell population was maintained in the expanded T cells (supplemental Figure 1A-B), which is consistent with a previous report.22
CLL patient T cells exhibit proliferative defects. (A) Comparison of the capacity of T cells to expand over time in adult patients with ALL (n = 7), MM (n = 23), and CLL (n = 12) during ex vivo expansion in response to CD3/CD28 stimulation. (B) Total number of lymphocyte population doublings in ALL and MM patients compared with CLL patients after 9 days of expansion, at which point T cells are harvested and banked for adoptive transfer into patients. **P < .01, ****P < .0001; error bars depict the standard error of the mean as determined by a 2-tailed Student t test. MM, multiple myeloma.
CLL patient T cells exhibit proliferative defects. (A) Comparison of the capacity of T cells to expand over time in adult patients with ALL (n = 7), MM (n = 23), and CLL (n = 12) during ex vivo expansion in response to CD3/CD28 stimulation. (B) Total number of lymphocyte population doublings in ALL and MM patients compared with CLL patients after 9 days of expansion, at which point T cells are harvested and banked for adoptive transfer into patients. **P < .01, ****P < .0001; error bars depict the standard error of the mean as determined by a 2-tailed Student t test. MM, multiple myeloma.
Long-term ibrutinib therapy repairs CLL T-cell defects that impair CTL019 cell production
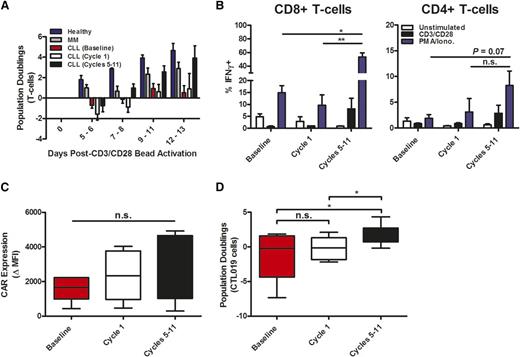
To examine the effects of ibrutinib therapy on the T-cell compartment in patients with CLL, we evaluated peripheral blood T lymphocytes from a cohort of patients with relapsed or refractory CLL during their course of treatment with single-agent ibrutinib. Clinical responses to this treatment are detailed in supplemental Table 1; most of these patients had partial responses to ibrutinib, with significant decreases in nodal mass burden but residual lymphocytosis. At baseline or after 1 cycle of ibrutinib treatment, CLL patient–derived T cells demonstrated minimal proliferation in response to CD3/CD28-bead stimulation (Figure 2A). However, this proliferative defect was completely reversed after 5 to 11 cycles of ibrutinib therapy, and T-cell proliferation kinetics resembled those of young, healthy donors and were slightly improved compared with older multiple myeloma patients (Figure 2A). Similarly, T-cell activation and secretion of interferon (IFN)-γ upon stimulation with CD3/CD28 beads and phorbol myristate acetate/ionomycin were improved after 5 to 11 cycles of treatment, particularly in the CD8 T-cell compartment (Figure 2B). Expression of the CAR was not different in T cells transduced after 5 to 11 cycles of ibrutinib conditioning (Figure 2C), but expansion after vector-mediated CAR transduction was significantly increased and more consistent (Figure 2D). Furthermore, exposure to CD19-expressing target cells induced a much greater proliferative response in CTL019 cells derived from CLL patients treated with 5 to 11 cycles of ibrutinib (supplemental Figure 2A). The functional importance of this capacity to proliferate after antigen receptor engagement is underscored by the finding that CTL019 cells manufactured from ibrutinib-naïve patients who did not respond to CTL019 treatment expanded poorly when exposed to CD19+ tumor targets in vitro, compared with patients who achieved a CR (supplemental Figure 2B). Accordingly, ex vivo proliferative potential upon antigen receptor stimulation appears to be consistent with the ability of CTL019 cells to expand when re-infused into patients (supplemental Figure 2C). The improved proliferative capacity of T cells from CLL patients who received long-term ibrutinib therapy is independent of both peripheral B-CLL tumor burden (supplemental Figure 3A-B) and the ratio of CD4+ to CD8+ T cells present before antigen receptor stimulation (supplemental Figure 3C-D). Thus, prolonged ibrutinib treatment of CLL patients reverses the defects in T-cell function, particularly proliferation in response to T-cell receptor stimulation.
Long-term oral ibrutinib therapy corrects functional defects in CLL patient T cells and enhances CTL019 cell generation. (A) Kinetics of CD3/CD28 bead–activated T-cell enrichment/expansion using static culture conditions in healthy donors (n = 5), MM patients (n = 5), and CLL patients at baseline, after cycle 1, and after 5 to 11 cycles of ibrutinib therapy (n = 10). (B) Frequency of IFN-γ-expressing CD8+ and CD4+ T cells shown in unstimulated, CD3/CD28−, and phorbol myristate acetate/ionomycin (PMA/Iono.)-stimulated ex vivo samples from CLL patients (n = 6) before and during ibrutinib therapy. (C) Efficiency of anti-CD19 CAR gene transfer into CLL patient T cells at baseline, after cycle 1, and after 5 to 11 cycles of ibrutinib treatment, expressed as the fold change in expression (geometric MFI) over matched untransduced samples (n = 6). (D) Maximum expansion of CTL019 cells generated from a longitudinal series of CLL patient T-cell samples at baseline, or after short-term and prolonged oral ibrutinib treatment (n = 10). Horizontal bars, boxes, and whiskers depict median, 25%/75% quartiles, and range, respectively. *P < .05, **P < .01; error bars represent the mean ± standard error as determined by a 2-tailed Student t test. MFI, mean fluorescence intensity; n.s., not statistically significant.
Long-term oral ibrutinib therapy corrects functional defects in CLL patient T cells and enhances CTL019 cell generation. (A) Kinetics of CD3/CD28 bead–activated T-cell enrichment/expansion using static culture conditions in healthy donors (n = 5), MM patients (n = 5), and CLL patients at baseline, after cycle 1, and after 5 to 11 cycles of ibrutinib therapy (n = 10). (B) Frequency of IFN-γ-expressing CD8+ and CD4+ T cells shown in unstimulated, CD3/CD28−, and phorbol myristate acetate/ionomycin (PMA/Iono.)-stimulated ex vivo samples from CLL patients (n = 6) before and during ibrutinib therapy. (C) Efficiency of anti-CD19 CAR gene transfer into CLL patient T cells at baseline, after cycle 1, and after 5 to 11 cycles of ibrutinib treatment, expressed as the fold change in expression (geometric MFI) over matched untransduced samples (n = 6). (D) Maximum expansion of CTL019 cells generated from a longitudinal series of CLL patient T-cell samples at baseline, or after short-term and prolonged oral ibrutinib treatment (n = 10). Horizontal bars, boxes, and whiskers depict median, 25%/75% quartiles, and range, respectively. *P < .05, **P < .01; error bars represent the mean ± standard error as determined by a 2-tailed Student t test. MFI, mean fluorescence intensity; n.s., not statistically significant.
Ibrutinib improves ex vivo CTL019 expansion, which is correlated with in vivo proliferation and clinical response
As part of the screening process for our CTL019 clinical protocol for CLL, a blood sample collected from subjects is tested for ex vivo expansion on a small scale to avoid full production efforts in patients with poor expansion kinetics. On this protocol, 67% of these patients achieved small-scale ex vivo expansion that is sufficient for embarking on full-scale production on the first attempt, which is lower than other diseases (and therefore, test expansion is not required). To examine the relationship between ex vivo expansion of T cells and efficacy of CTL019 in CLL patients, we compared the proliferative burst of T cells and CTL019 cells in ibrutinib-naïve CLL patients who achieved a CR, a partial response, or no response to CTL019 treatment; the clinical history and outcomes of these patients have been described.11 Separately, we analyzed the proliferative capacities of T cells and CTL019 cells from 3 CLL patients who had extensive prior treatment with ibrutinib. The treatment history and responses of those patients who received long-term ibrutinib therapy before T-cell collection for CTL019 therapy are summarized in Table 1. Small-scale expansions of T cells from ibrutinib-naïve patients who ultimately achieved CRs and those who had extensive prior ibrutinib treatment history tended to have the highest ex vivo proliferative capacity (similar to T cells from adult patients with ALL), whereas ibrutinib-naïve nonresponders to CTL019 displayed a reduced proliferative potential ex vivo (Figure 3A); this was also true of vector-transduced CAR T cells generated from these subjects (Figure 3B). Ibrutinib-pretreated CLL patients who were infused with CTL019 cells also exhibited robust engraftment in the peripheral blood (Figure 3C; supplemental Figure 4A) and bone marrow (supplemental Figure 4B). Engraftment was accompanied by clinical responses (Table 1; supplemental Figure 4C). CTL019 in vivo function in ibrutinib-treated subjects was similar to CTL019 function in ibrutinib-naïve CLL patients who achieved CRs (Figure 3C). Importantly, we also found that the ex vivo proliferative ability of CTL019 cells was a predictive indicator of their engraftment level in vivo after adoptive transfer (Figure 3D). On our CTL019 treatment protocols, all other antitumor therapy had to be discontinued before CTL019 infusion and, therefore, we could account for only the effects of ibrutinib as a prior exposure. Thus, these data indicate that improvements in ex vivo T-cell function correlate with in vivo CAR T-cell function, which is required for clinical benefit from CAR T-cell therapies.
Demographics and responses of patients who received ibrutinib before CAR T-cell therapy
| Subject UPN . | Age, y/sex . | Previous therapies . | CLL tumor characteristics . | Ibrutinib status during T-cell collection . | CLL involvement at CTL019 infusion . | Response to CTL019 . |
|---|---|---|---|---|---|---|
| 18 | 61/male | Fludarabine/cyclophosphamide/rituximab × 6 cycles Bendamustine/rituximab × 1 cycle Oxaliplatin/fludarabine/cytarabine/rituximab × 2 cycles Selinexor × 1 cycle Ibrutinib × 12 mo | trisomy 12; del12q14; del11q; del17p | Discontinued 2 mo before T-cell collection | Cytopenias; BM 20% involved with CLL; bulky diffuse adenopathy | Marked PR; cytopenias resolved at 2 mo; BM minimally involved; >50% decrease in adenopathy; relapsed with CD19-negative disease |
| 29 | 68/male | Rituximab/fludarabine × 6 cycles Ofatumumab × 2 cycles Bendamustine/ofatumumab × 1 cycle Ibrutinib × 15 mo | 13qdel; trisomy 21; 17pdel | Discontinued 11 d before T-cell collection | Blood involved; BM 25% involved with CLL; diffuse adenopathy | PR: cleared blood and BM at day 28; persistent adenopathy |
| 47 | 57/male | Fludarabine/cyclophosphamide/rituximab × 3 cycles Bendamustine × 1 cycle Ibrutinib × 16 mo | p53del | Ongoing at T-cell collection*; discontinued 7 d before CTL019 treatment, per protocol | Normal ALC; BM 70% involved with CLL; diffuse adenopathy | CR: cleared blood and BM at day 28; no adenopathy |
| Subject UPN . | Age, y/sex . | Previous therapies . | CLL tumor characteristics . | Ibrutinib status during T-cell collection . | CLL involvement at CTL019 infusion . | Response to CTL019 . |
|---|---|---|---|---|---|---|
| 18 | 61/male | Fludarabine/cyclophosphamide/rituximab × 6 cycles Bendamustine/rituximab × 1 cycle Oxaliplatin/fludarabine/cytarabine/rituximab × 2 cycles Selinexor × 1 cycle Ibrutinib × 12 mo | trisomy 12; del12q14; del11q; del17p | Discontinued 2 mo before T-cell collection | Cytopenias; BM 20% involved with CLL; bulky diffuse adenopathy | Marked PR; cytopenias resolved at 2 mo; BM minimally involved; >50% decrease in adenopathy; relapsed with CD19-negative disease |
| 29 | 68/male | Rituximab/fludarabine × 6 cycles Ofatumumab × 2 cycles Bendamustine/ofatumumab × 1 cycle Ibrutinib × 15 mo | 13qdel; trisomy 21; 17pdel | Discontinued 11 d before T-cell collection | Blood involved; BM 25% involved with CLL; diffuse adenopathy | PR: cleared blood and BM at day 28; persistent adenopathy |
| 47 | 57/male | Fludarabine/cyclophosphamide/rituximab × 3 cycles Bendamustine × 1 cycle Ibrutinib × 16 mo | p53del | Ongoing at T-cell collection*; discontinued 7 d before CTL019 treatment, per protocol | Normal ALC; BM 70% involved with CLL; diffuse adenopathy | CR: cleared blood and BM at day 28; no adenopathy |
ALC, absolute lymphocyte count; BM, bone marrow; PR, partial response; UPN, unique patient number.
Modified CTL019 manufacturing process.
Absolute lymphocyte count.
Ibrutinib potentiates CTL019 cell expansion and engraftment, which correlates with clinical response. Ex vivo proliferation of total T cells during test expansion (A) and of CTL019 cells during clinical manufacturing (evaluated at day 9 after CD3/CD28-induced activation) (B) derived from patients with CLL and stratified by clinical response (n = 16 no response [NR], n = 7 partial response [PR], n = 7 CR) and by treatment (n = 3 pretreated with ibrutinib) compared with adults with ALL (n = 7). (C) In vivo engraftment of autologous CTL019 cells from the above CLL patients expressed as maximum expansion of adoptively transferred lymphocytes (copies of anti-CD19 CAR per microgram of genomic [g]DNA) in the first 90 days postinfusion. (D) Spearman ρ correlation shown between ex vivo expansion potential and in vivo proliferative capacity (maximum copies of anti-CD19 CAR per microgram of gDNA within 90 days after infusion) of adoptively transferred CTL019 cells in matched CLL patients (n = 30). *P < .05, **P < .01; error bars show mean ± standard error as determined by a 2-tailed Student t test. n.s., not statistically significant.
Ibrutinib potentiates CTL019 cell expansion and engraftment, which correlates with clinical response. Ex vivo proliferation of total T cells during test expansion (A) and of CTL019 cells during clinical manufacturing (evaluated at day 9 after CD3/CD28-induced activation) (B) derived from patients with CLL and stratified by clinical response (n = 16 no response [NR], n = 7 partial response [PR], n = 7 CR) and by treatment (n = 3 pretreated with ibrutinib) compared with adults with ALL (n = 7). (C) In vivo engraftment of autologous CTL019 cells from the above CLL patients expressed as maximum expansion of adoptively transferred lymphocytes (copies of anti-CD19 CAR per microgram of genomic [g]DNA) in the first 90 days postinfusion. (D) Spearman ρ correlation shown between ex vivo expansion potential and in vivo proliferative capacity (maximum copies of anti-CD19 CAR per microgram of gDNA within 90 days after infusion) of adoptively transferred CTL019 cells in matched CLL patients (n = 30). *P < .05, **P < .01; error bars show mean ± standard error as determined by a 2-tailed Student t test. n.s., not statistically significant.
Prolonged ibrutinib treatment decreases immunosuppressive PD-1 and CD200 expression
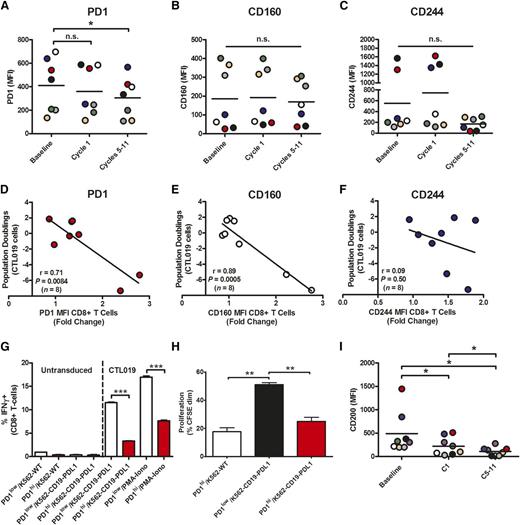
To evaluate the potential mechanisms of ibrutinib-mediated repair of T-cell function in CLL, we examined the phenotype of CD8+ T cells in the blood of CLL patients during their course of treatment with ibrutinib. T cells from CLL patients are known to exhibit certain features of exhaustion, including abnormally high levels of PD-1, CD160, and CD244,23 which are often overexpressed in chronic viral infections. We noted a significant decrease in the expression level (mean fluorescence intensity) of PD-1 after 5 to 11 cycles of ibrutinib (Figure 4A), which was independent of changes in the activation state of these cells (supplemental Figure 5A). Prolonged treatment with ibrutinib, however, did not significantly affect the expression of 2 other inhibitory receptors, CD160 (Figure 4B) and CD244 (Figure 4C), in any of the patient samples we evaluated. Notably, in the instances in which there was increased expression of a particular inhibitory receptor (be it PD-1, CD160, or CD244) at baseline, extended ibrutinib treatment lowered the level of the elevated inhibitory molecule (Figure 4A-C). In contrast, T-cell memory phenotype (supplemental Figure 5B) and ex vivo apoptosis sensitivity (supplemental Figure 5C) did not differ from baseline. The expression levels of PD-1 (Figure 4D; supplemental Figure 6A-B) and CD160 (Figure 4E), but not CD244 (Figure 4F), on T cells from CLL patients negatively correlated with the proliferative capacity of these lymphocytes during ex vivo expansion. A significant negative correlation between the frequency of PD-1–expressing T cells and their ability to double in response to antigen receptor stimulation was also seen (supplemental Figure 6C). It is of interest, however, that prolonged ibrutinib therapy did not affect the percentages of PD-1+CD8+ T cells (supplemental Figure 6D). Similarly, the frequencies of CTLA4 surface–positive T cells (supplemental Figure 6E) and CD80/CD86-bearing B-cell targets (supplemental Figure 6F-G) were unaffected by long-term ibrutinib treatment.
Prolonged ibrutinib therapy decreases levels of inhibitory molecules on T cells and B-CLL cells. Expression levels of PD-1 (A), CD160 (B), and CD244 (C) on ex vivo CD8+ T cells at baseline and during the course of ibrutinib treatment in patient samples (n = 7). (D-F) Pearson correlations shown between the ex vivo proliferative capacity of CTL019 cells and the expression levels of PD-1 (D), CD160 (E), and CD244 (F). Inhibitory molecule levels on baseline CLL patient (n = 8) CD8+ T cells after anti-CD19 CAR gene transfer are depicted as a fold change in expression over a biological standard. (G) Proportion of IFN-γ-producing untransduced, CTL019, or PD-1-expressing CTL019 cells generated from healthy subjects and incubated for 6 hours with the indicated stimuli. (H) Proliferative capacity (assessed at day 6) of carboxyfluorescein succinimidyl ester (CFSE)–labeled healthy donor CTL019 cells (n = 3) after PD-1 overexpression and coculture with the indicated tumor targets. (I) Expression levels of CD200 on B-CLL cells in CLL patients (n = 8) at baseline, after 1 cycle (C1), and after 5 to 11 cycles (C5-11) of oral ibrutinib therapy. Each colored circle represents an individual patient. Significant differences in expression levels as determined by 2-tailed Student t test are depicted as *P < .05, **P < .01, and ***P < .001. n.s., not statistically significant.
Prolonged ibrutinib therapy decreases levels of inhibitory molecules on T cells and B-CLL cells. Expression levels of PD-1 (A), CD160 (B), and CD244 (C) on ex vivo CD8+ T cells at baseline and during the course of ibrutinib treatment in patient samples (n = 7). (D-F) Pearson correlations shown between the ex vivo proliferative capacity of CTL019 cells and the expression levels of PD-1 (D), CD160 (E), and CD244 (F). Inhibitory molecule levels on baseline CLL patient (n = 8) CD8+ T cells after anti-CD19 CAR gene transfer are depicted as a fold change in expression over a biological standard. (G) Proportion of IFN-γ-producing untransduced, CTL019, or PD-1-expressing CTL019 cells generated from healthy subjects and incubated for 6 hours with the indicated stimuli. (H) Proliferative capacity (assessed at day 6) of carboxyfluorescein succinimidyl ester (CFSE)–labeled healthy donor CTL019 cells (n = 3) after PD-1 overexpression and coculture with the indicated tumor targets. (I) Expression levels of CD200 on B-CLL cells in CLL patients (n = 8) at baseline, after 1 cycle (C1), and after 5 to 11 cycles (C5-11) of oral ibrutinib therapy. Each colored circle represents an individual patient. Significant differences in expression levels as determined by 2-tailed Student t test are depicted as *P < .05, **P < .01, and ***P < .001. n.s., not statistically significant.
We also examined how PD-1, CD160, and CD244 expression was modulated during the time course of CD3/CD28-induced T-cell expansion. In line with the kinetics of expansion after activation (supplemental Figure 7A), the percentages of PD-1+, CD160+, and CD244+ T cells peaked at day 5, followed by a decline to reach steady-state frequencies (supplemental Figure 7B). In addition, CD3/CD28-mediated T-cell expansion kinetics coincided with increased PD-1 expression, but not upregulated CD160 or CD244 levels (supplemental Figure 7C). These data likely reflect a transient upregulation of PD-1 that is associated with T-cell activation rather than chronic exhaustion.
We next wanted to develop a system to directly evaluate the effect of PD-1 ligation by natural ligands on CAR T-cell activation. Previously reported assays have involved the use of agonistic antibodies and/or pervanadate.24-27 In addition, many gene transfer methods require preactivation of cells, which hampers the study of lymphocytes transitioning from G0 to G1, a likely central target of PD-1–induced inhibition.28,29 We therefore transfected resting, healthy-donor T cells with CAR mRNA with or without PD-1 mRNA. High and uniform expression of anti-CD19 CAR was observed over time (supplemental Figure 8A), demonstrating that RNA transfection is an efficient way to generate CAR T cells for signaling and functional investigations. In contrast, PD-1 expression was more transient, with markedly decreased levels by day 6 posttransfection (supplemental Figure 8A). Decreased IFN-γ production (Figure 4G; supplemental Figure 8B), IL-2 secretion (supplemental Figure 8C), and proliferation (Figure 4H; supplemental Figure 8D) were observed upon coculture of T cells expressing high levels of PD-1 (PD-1hi) with CD19+ target cells bearing the PD-1 natural ligand, PD-L1. The immunosuppressive effects of transfection-mediated PD-1 expression were inhibited by blocking PD-1 ligation (supplemental Figure 8B-D). These data indicate that PD-1 ligation delivers an inhibitory signal to CAR-mediated T-cell activation. Of interest, PD-L1 checkpoint blockade has been shown to normalize the T-cell compartment in the Eμ-TCL1 mouse model of CLL30 ; however, even though PD-1 expression in CAR T cells in vivo has been observed,8 its physiologic effects on the T-cell compartment in CLL or in CAR T cells in CLL has not yet been elucidated.
Another major determinant of the ability of CTL019 cells to expand after adoptive transfer is the degree of tumor-mediated suppression through the binding of inhibitory ligands. In particular, signals delivered through the CD200/CD200 receptor (CD200R) axis have been shown to play an important role in the regulation of antitumor immunity,31-33 with CD200 overexpression in CLL leading to the functional impairment of CD8+ T-cell responses.34 Because CLL cells express high levels of CD200 and because CD200/CD200R interactions may be an important pathway involved in T-cell suppression, we hypothesized that BTK signaling in CLL cells may alter the expression of this major inhibitory ligand. Therefore, the effect of oral ibrutinib therapy on CD200 expression by B-CLL cells was examined. We noted a marked decrease in expression of CD200 during ibrutinib treatment (Figure 4I), indicating that ibrutinib may also have indirect effects on the T-cell compartment by modulating T cell–inhibiting molecules on CLL cells independently of the PD-L1/PD-1 axis.
Ex vivo ibrutinib exposure does not alter CTL019 function
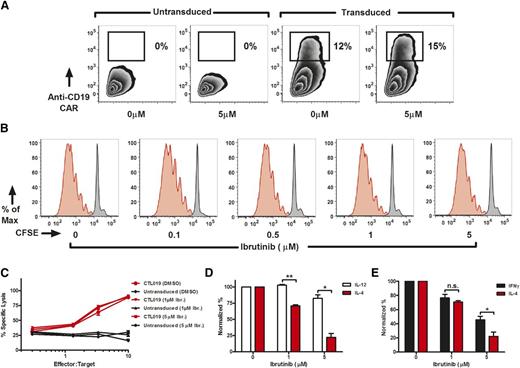
We have previously demonstrated that ibrutinib-mediated ITK inhibition inhibits Th2-type polarization in purified CD4+ T-cell cultures, thereby shifting the balance between Th2 and Th1 cells.17 Although we could not measure the effect of continued ibrutinib exposure in patients who had received CTL019, we evaluated the effect of concurrent ibrutinib treatment in vitro and in xenogeneic mouse models. For in vitro experiments, we briefly exposed T cells to ibrutinib and then performed a wash to allow only for specific and irreversible binding to ITK, rather than nonspecific kinase inhibition.17,35,36 Supraphysiological doses of ibrutinib did not impair or enhance transduction with the anti-CD19 CAR lentiviral vector (Figure 5A), and there was no effect on T-cell proliferation (Figure 5B). Similarly, pretreatment of CAR T cells with high concentrations of ibrutinib followed by washout before coculture with target CD19-expressing leukemic cells did not impact cytolytic function (Figure 5C). At a high concentration of ibrutinib, the Th1 skewing mediated by ITK inhibition of ibrutinib became evident in the cytokine profile measured after co-incubation of ibrutinib-pretreated CTL019 cells with CD19+ targets; under these conditions, IL-4 production was selectively inhibited compared with production of IL-12 (Figure 5D) and IFN-γ (Figure 5E). Thus, despite the fact that ibrutinib irreversibly inhibits ITK in T cells, a pulse dose of ibrutinib did not alter T-cell proliferation or CAR T cell–mediated cytotoxicity in vitro, even at concentrations of ibrutinib that exceed those used clinically.
Ibrutinib does not impair CAR gene transfer, T-cell proliferation, or cytotoxic capacity and limits Th2 activation in CTL019 cells. (A) Anti-CD19 CAR lentiviral transduction efficiency after T-cell enrichment/expansion in the presence or absence of ibrutinib (0-5 μM). (B) Proliferative capacity of T cells as demonstrated by a CFSE dilution assay in unstimulated (black peaks) and CD3/CD28 bead–stimulated (red peaks) lymphocytes in the presence or absence of short-term ibrutinib exposure (0-5 μM). Experiments were repeated at least 3 times with independent donors. Culture conditions were set up to mimic the expansion process used to produce CTL019 cells. (C) Cytotoxic capacity of CTL019 cells after overnight coculture with tumors. For cytokine analysis and the cytotoxicity assays, CAR T cells were pretreated with ibrutinib for 1 hour and washed extensively before exposure to targets. The cytotoxicity assay is representative of 4 independent experiments conducted with different healthy donors. Normalized production of IL-12 (D) and IFN-γ (E) compared with IL-4 (n = 3 independent donors) in expanded CTL019 cells exposed for 18 hours to targets expressing K562-CD19. Significant differences are depicted as *P < .05 and **P < .01 (2-tailed Student t test). DMSO, dimethylsulfoxide; Ibr., ibrutinib; n.s., not statistically significant.
Ibrutinib does not impair CAR gene transfer, T-cell proliferation, or cytotoxic capacity and limits Th2 activation in CTL019 cells. (A) Anti-CD19 CAR lentiviral transduction efficiency after T-cell enrichment/expansion in the presence or absence of ibrutinib (0-5 μM). (B) Proliferative capacity of T cells as demonstrated by a CFSE dilution assay in unstimulated (black peaks) and CD3/CD28 bead–stimulated (red peaks) lymphocytes in the presence or absence of short-term ibrutinib exposure (0-5 μM). Experiments were repeated at least 3 times with independent donors. Culture conditions were set up to mimic the expansion process used to produce CTL019 cells. (C) Cytotoxic capacity of CTL019 cells after overnight coculture with tumors. For cytokine analysis and the cytotoxicity assays, CAR T cells were pretreated with ibrutinib for 1 hour and washed extensively before exposure to targets. The cytotoxicity assay is representative of 4 independent experiments conducted with different healthy donors. Normalized production of IL-12 (D) and IFN-γ (E) compared with IL-4 (n = 3 independent donors) in expanded CTL019 cells exposed for 18 hours to targets expressing K562-CD19. Significant differences are depicted as *P < .05 and **P < .01 (2-tailed Student t test). DMSO, dimethylsulfoxide; Ibr., ibrutinib; n.s., not statistically significant.
Continuous ibrutinib treatment enhances CTL019 efficacy in drug-resistant ALL and CLL mouse models
Prolonged ibrutinib treatment of CLL patients appeared to enhance CAR T-cell manufacturing and engraftment, whereas a single cycle of ibrutinib treatment or a brief pulse of in vitro ibrutinib exposure had minimal effects on CAR T-cell function. We next hypothesized that continued ibrutinib exposure could have effects on CAR T-cell function in vivo. We could not test the effect of continued ibrutinib therapy in patients who were treated with CAR T cells, because discontinuation of other tumor-directed therapies was required per these clinical protocols, which have completed accrual. Therefore, we tested the combination of continuous ibrutinib treatment with CAR T cells in 2 xenograft models of B-cell leukemia (Figure 6A).
Ibrutinib increases the engraftment and antitumor activity of CTL019 cells when administered concurrently. (A) Protocol schema depicting the engraftment of NOD scid gamma (NSG) mice with 106 luciferase-expressing Nalm-6 cells or 5 to 10 × 106 OSU-CLL cells. At day 7 or 9, animals (n = 5-12 per group) were randomized according to tumor burden to receive vehicle alone, 25 mg/kg per day of ibrutinib, or 106 CTL019 cells per mouse. Ibrutinib or empty vehicle was continuously administered for the entire duration of animal experiments. (B) Absolute numbers of adoptively transferred peripheral blood T cells were monitored weekly by retro-orbital bleeding and flow cytometric detection. (C) Bioluminescence images of 5 representative mice in the CTL019 and CTL019 + Ibr. treatment groups are shown at day 20 post–CAR T-cell infusion. (D) Peak bioluminescent (BLI) tumor burden depicted for each experimental group. (E) PD-1 expression on adoptively transferred CTL019 cells in the spleens of ibrutinib-naive or ibrutinib-treated mice at the time of euthanasia. (F) Overall survival of untreated mice (Nalm-6 alone [No Rx]) or animals treated with ibrutinib alone, CTL019 cells, or a combination of CTL019 and ibrutinib shown over time. Two separate experiments with similar results were performed. (G) In vivo expansion kinetics of CTL019 cells in mice with or without continuous ibrutinib treatment. Error bars show the standard error. (H) Survival of OSU-CLL-bearing NSG mice treated on day 7 after tumor injection with 106 CTL019 cells (n = 12 mice per group). Overall survival curves were plotted using the Kaplan-Meier method and compared using the log-rank (Mantel-Cox) test. Statistical significance between groups with differences in CTL019 expansion and tumor burden was determined using a 2-tailed Student t test. *P < .05, **P < .01, ***P < .001, ****P < .0001. CBG, click beetle green; n.s., not statistically significant.
Ibrutinib increases the engraftment and antitumor activity of CTL019 cells when administered concurrently. (A) Protocol schema depicting the engraftment of NOD scid gamma (NSG) mice with 106 luciferase-expressing Nalm-6 cells or 5 to 10 × 106 OSU-CLL cells. At day 7 or 9, animals (n = 5-12 per group) were randomized according to tumor burden to receive vehicle alone, 25 mg/kg per day of ibrutinib, or 106 CTL019 cells per mouse. Ibrutinib or empty vehicle was continuously administered for the entire duration of animal experiments. (B) Absolute numbers of adoptively transferred peripheral blood T cells were monitored weekly by retro-orbital bleeding and flow cytometric detection. (C) Bioluminescence images of 5 representative mice in the CTL019 and CTL019 + Ibr. treatment groups are shown at day 20 post–CAR T-cell infusion. (D) Peak bioluminescent (BLI) tumor burden depicted for each experimental group. (E) PD-1 expression on adoptively transferred CTL019 cells in the spleens of ibrutinib-naive or ibrutinib-treated mice at the time of euthanasia. (F) Overall survival of untreated mice (Nalm-6 alone [No Rx]) or animals treated with ibrutinib alone, CTL019 cells, or a combination of CTL019 and ibrutinib shown over time. Two separate experiments with similar results were performed. (G) In vivo expansion kinetics of CTL019 cells in mice with or without continuous ibrutinib treatment. Error bars show the standard error. (H) Survival of OSU-CLL-bearing NSG mice treated on day 7 after tumor injection with 106 CTL019 cells (n = 12 mice per group). Overall survival curves were plotted using the Kaplan-Meier method and compared using the log-rank (Mantel-Cox) test. Statistical significance between groups with differences in CTL019 expansion and tumor burden was determined using a 2-tailed Student t test. *P < .05, **P < .01, ***P < .001, ****P < .0001. CBG, click beetle green; n.s., not statistically significant.
In a xenograft mouse model bearing human ALL (Nalm-6) cells, CTL019 therapy or ibrutinib alone had minimal effects in the setting of very advanced disease (ie, delayed administration of CAR T cells). However, coadministration of ibrutinib with CTL019 resulted in significantly increased T-cell expansion in the peripheral blood (Figure 6B) and lower tumor burden (Figure 6C-D). In accordance with our data in ibrutinib-treated patients, we observed a significantly lower level of PD-1 expression on adoptively transferred T cells when mice received concurrent ibrutinib therapy (Figure 6E). In contrast, ibrutinib treatment did not affect CD200 levels on the leukemic cell line (supplemental Figure 9A), which may be attributed to the fact that the animals were not engrafted with ibrutinib-sensitive tumor cells. Tumor-bearing animals that received CTL019 cells with continuous ibrutinib had improved survival (Figure 6F). Therefore, in this ibrutinib-resistant model of ALL, concurrent ibrutinib therapy with CTL019 successfully converted a model that was lethal with either single-agent therapy into a curative one with combination therapy.
We next tested the efficacy of combining CTL019 and ibrutinib in a more relevant model of CLL using OSU-CLL cells. This cell line has the immunophenotype and migratory properties similar to the primary CLL tumor from which it was derived.19 In this system, based on the engraftment of 5 to 10 million B-CLL cells, neither single-agent ibrutinib nor CTL019 affected overall survival. In contrast, the combination of these agents resulted in both markedly enhanced CTL019 engraftment in the peripheral blood (Figure 6G) and significant prolongation of survival (Figure 6H). This enhanced effect of ibrutinib on CTL019 therapy was not attributed to allogeneic T-cell responses (supplemental Figure 9B). Although it is possible that the observed increase in the peripheral blood engraftment of CAR T cells is a function of ibrutinib-mediated changes in lymphocyte chemotaxis and adhesion, the improvements in tumor burden and survival argue for an overall increase in the efficacy of CAR T-cell function.
Discussion
The feasibility and efficacy of potentially curative CAR T-cell therapy to the CLL patient population is likely to be enhanced by strategies that repair the T-cell defect manifested here as poor expansion both ex vivo and in vivo.11,23 Herein, we have presented evidence that although short-term treatment with ibrutinib did not enhance T-cell function in CLL patients or in vitro, long-term ibrutinib therapy for at least 5 months restored T-cell activity to levels comparable to young, healthy donors with respect to effective ex vivo expansion of CAR-T cells. Furthermore, a small cohort of 3 CLL patients who were treated with single-agent ibrutinib for at least 1 year in the weeks before T-cell collection had robust T-cell and CAR T-cell proliferation and function both ex vivo and in vivo. One of these patients (UPN 18), who was treated with single-agent ibrutinib for 12 months before T-cell collection, had remarkable ex vivo and in vivo T-cell expansions. After infusion of CTL019 cells, he attained a very good partial response for 6 months but relapsed with a CD19-negative plasmablastic lymphoma and evidence of a small clone of CD19-negative CLL. The successful collection of CTL019 cells from this individual, despite heavy pretreatment, his robust response, and the emergence of CD19-negative relapse, suggested highly effective CTL019 cellular therapy. Notably, ibrutinib treatment was able to overcome the long-term immunosuppressive effects of the underlying disease and traditional CLL therapies, which may otherwise hamper autologous adoptive T-cell transfer approaches. Although these results are promising, evaluation of a larger cohort of patients is required to make more definitive conclusions regarding the potentiation of CAR T-cell clinical efficacy by ibrutinib treatment.
In this study, we demonstrated that ibrutinib treatment enhances the feasibility of generating CAR T cells from CLL patients. Identifying the mechanisms by which this occurs could allow for more precise therapeutic targeting of relevant pathways, which may also have applicability to other cancers. The increased expression of inhibitory receptors such as PD-1, CD160, and CD244 on CLL T cells likely contributes to impaired T-cell function.23 Abnormally high expression of CD160 and PD-1 is of particular interest, based on recent reports of their respective ligands (herpesvirus-entry mediator and PD-L1) harboring mutations and/or gene fusions in the context of other B-cell malignancies.37,38 We found that 5 to 11 cycles of ibrutinib therapy led to a significant decrease in the levels of PD-1 expression on T cells from CLL patients compared with baseline and cycle 1 time points. Of interest, we noted that in all instances in which there was elevation of PD-1, CD160, or CD244 levels at baseline, long-term ibrutinib therapy lowered the expression of the respective inhibitory receptor. These data suggest that prolonged ibrutinib treatment may change the inhibitory phenotype of CD8+ T cells in CLL through a common pathway.
Continuous ibrutinib therapy also resulted in diminished PD-1 expression on adoptively transferred T cells in an animal model of resistant leukemia. Furthermore, PD-1 levels on CD8+ T cells were inversely correlated with the ability to generate and expand CTL019 cells from baseline lymphocyte samples, suggesting that this inhibitory pathway is potentially associated with the poor proliferative capacity of T cells in CLL disease. Similarly, proliferation of CAR T cells bearing high levels of PD-1 was inhibited by tumor targets bearing its natural ligand, PD-L1, indicating the relevance of the PD-1/PD-L1 axis in the setting of CAR T-cell therapy. There was also a negative correlation between CD160 expression and the proliferative capacity of CTL019 cells, although ibrutinib treatment did not affect expression levels of this inhibitory receptor. It will be interesting to determine in future studies whether PD-1 and CD160 coexpression leads to an advanced state of T-cell exhaustion in CLL, which has been previously reported in chronic viral infections.39
It is now becoming increasingly recognized that cancer cells actively use immune evasion mechanisms to suppress T-cell function, and this clearly represents a major biologic barrier to the success of immunotherapy, including adoptive cell transfer. We therefore wanted to gain insight into the molecular targets of tumor-induced T-cell dysregulation before and during the course of oral ibrutinib therapy. It has been previously shown that CD200 is uniformly expressed in CLL, with high levels serving as a predictive indicator of poor prognosis in this disease and others.40,41 We demonstrated that expression levels of CD200 on B-CLL cells were progressively diminished with successive cycles of ibrutinib treatment. CD200-mediated ligation of CD200R42 is known to deliver an inhibitory signal to the target cell.43-45 Consistent with these reports, upregulation of CD200 on CLL cells is sufficient to downregulate Th1 immunity, including cytokines such as IL-2 and IFN-γ.42 In addition, Pallasch et al demonstrated that CD200 expression by B-CLL cells had direct inhibitory effects on the proliferation of autologous effector T cells.46 Furthermore, blockade of CD200, as well as of PD-L1, reverses the functional impairment of T lymphocytes by B-CLL cells.47 Because ibrutinib therapy did not influence the differentiation state of T cells or their apoptosis sensitivity, the observed functional improvements may be attributed to the decreased expression levels of inhibitory molecules that we report here and to the consequent reversal of tumor/T-cell inhibitory signaling. Indeed, CD200, PD-1, and the pathways that control their activity may provide new opportunities to reduce T-cell immunosuppression and improve therapeutic cellular products for the treatment of hematologic malignancies.
Ibrutinib was recently identified as the first clinically viable, irreversible ITK inhibitor that enhances Th1-based immunity by a selective inhibition of Th2 responses.17 Our in vitro studies demonstrated that CAR T-cell function is unimpaired by ibrutinib, as illustrated by intact proliferative capacity, tumor recognition, and cytotoxicity. Short-term exposure of CTL019 cells to ibrutinib during coculture with ibrutinib-resistant CD19+ tumor targets resulted in minimal Th1 skewing only at supraphysiological concentrations not obtained in clinical practice. These observations are supported by our evaluations of primary CLL patient T cells in which there are no apparent functional benefits associated with acute periods of ibrutinib treatment, and the finding that extended therapy to at least 5 months is required for enhancement of CTL019 cell generation. Together, these data suggest that ibrutinib repairs the T-cell compartment in CLL in part by an indirect effect, whereby inhibition of BTK signaling and modulation of B-CLL inhibitory ligand expression and disease leads to relief of T-cell immunosuppression. Our ongoing studies aim to determine whether ibrutinib-mediated Th2-to-Th1 skewing effects may be enhanced in CAR-redirected, purified populations of CD4+ T cells.
Although ibrutinib therapy improves symptoms and survival in CLL, it is not a curative therapy and requires continuous treatment for life. In addition, a subset of patients with a complex karyotype is at most risk for progressive disease on ibrutinib, either as Richter transformation or as relapsed CLL that occurs with the C481S or PLCγ2 mutations.48 Given that 90% or more of patients with high-risk disease have an initial response to ibrutinib and typically remain on ibrutinib therapy for over 1 year, this time window may provide an optimal opportunity to collect T cells and to subsequently administer a potentially curative T cell–based therapy that would allow for cessation of drug treatment. Furthermore, the effects of ibrutinib on lymphocyte chemotaxis, which is clinically manifested as rapid and sustained reduction in lymphadenopathy accompanied by lymphocytosis,49 may facilitate CAR T-cell engraftment and function, either by directly affecting the function of T cells or by facilitating exposure to tumor cells in circulation. Of interest, CTL019 appears to clear the bone marrow and peripheral blood compartments more rapidly and effectively than lymph nodes, indicating a different migration profile for CTL019 cells than for CLL cells in the absence of ibrutinib.
We also established that concurrent administration of ibrutinib can improve CAR T-cell efficacy in murine ALL and CLL models. The enhanced antitumor effect of CAR T cells by concurrent ibrutinib therapy was accompanied by a significant reduction in PD-1 expression by CAR T cells transferred into xenogeneic leukemia systems, independently of changes in CD200 expression in these ibrutinib-resistant models. This is a novel finding in the context of CAR T-cell therapy and complements recent reports of ibrutinib demonstrating positive immunomodulatory effects in other mouse models of cancer, where it enhances T-cell responses to adjuvants in lymphoma and to the checkpoint inhibitors PD-1/PD-L1 in solid tumors such as colon and breast cancer.18,50 This finding also suggests that repair of the T-cell compartment by ibrutinib may improve endogenous anti-CLL responses. Thus, ibrutinib can sculpt antitumor immunity mediated by adoptively transferred CAR T cells and augment the effectiveness of that response. Whether this occurs solely through changes in inhibitory receptor expression such as the PD-1/PD-L1 axis or occurs through additional mechanisms remains an active area of investigation.
In contrast to our findings in ibrutinib-treated patients, we did not observe that CD200 levels were reduced on leukemia cells in vivo by ibrutinib therapy. This observation may be attributed to the fact that we were not able to evaluate the combination of ibrutinib with CAR T cells in a xenogeneic model of ibrutinib-sensitive leukemia. Although this may be the more relevant setting, because most patients with CLL have ibrutinib-sensitive disease (ie, accompanied by CD200 downregulation on B-CLL cells), appropriate cell lines for xenogeneic modeling are not currently available. Therefore, future studies are needed to investigate the mechanisms underlying the release of immunosuppression afforded by ibrutinib in the context of T-cell mediated antitumor immunity.
In summary, we have shown that long-term ibrutinib treatment reverses the dysfunction of T cells in CLL, which has implications for both the mechanism of action of ibrutinib and the potential use of T-cell therapies. The enhanced ex vivo proliferative capacity of CAR-redirected lymphocytes is a strong predictive indicator of their ability to engraft in vivo and mediate antitumor responses. This increased therapeutic potential is further supported by the synergistic effect of CAR T cells and ibrutinib in resistant models of acute and chronic leukemia. These data suggest that clinical trials with ibrutinib lead-in and then continued treatment could enhance the efficacy and engraftment of adoptively transferred T cells, including CAR T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jason A. Dubovsky (Ohio State University) and Fang Chen, Olga Shestova, Saad Kenderian, Alexander Malykhin, and Jihyun Lee (University of Pennsylvania) for experimental assistance; Amber Gordon (Ohio State University) and Vanessa Gonzalez, Holly McConville, and Edward Pequignot (University of Pennsylvania) for help with patient sample analysis; and Brian Keith and Whitney Gladney (University of Pennsylvania) for their helpful suggestions.
This work was supported by the National Institutes of Health National Cancer Institute grants T32 CA009140 (J.A.F.), K08 CA166039 (M.V.M.), P01 CA095426 (N.M. and J.C.B.), K23 CA178183 (J.A.W.), R35 CA198183 (J.C.B.), R01 CA177292 (J.C.B.), and R01 CA165206 (C.H.J. and D.L.P.); the D. Warren Brown Foundation; the Four Winds Foundation (J.C.B.); the Lymphoma Research Foundation (M.R.); and Novartis.
Authorship
Contribution: J.A.F., K.A.B., P.R.P., Z.Z., M.R., D.M.B., S.E.M., D.R.C., C.Z., J.X., J.H., S.B.K., and A.P.C. performed the experiments; J.A.F., K.A.B., S.F.L., J.J.M., S.G., D.L.P., B.L.L., and M.V.M. analyzed the results and created the figures; J.A.F., K.A.B., P.D., J.A.W., M.L., A.J.J., K.M., N.M., C.H.J., J.C.B., and M.V.M. designed the research; and J.A.F., K.A.B., J.C.B., and M.V.M. wrote and edited the paper.
Conflict-of-interest disclosure: The University of Pennsylvania has entered into an alliance with Novartis Pharmaceuticals to develop CAR T cells as a platform for multiple cancers. J.A.F., K.A.B., M.R., D.L.P., N.M., C.H.J., J.C.B., and M.V.M. hold patents related to the work that is described in the present study. The remaining authors declare no competing financial interests.
Correspondence: Marcela V. Maus, Massachusetts General Hospital Cancer Center, Building 149, Thirteenth St, Charlestown, MA 02129; e-mail: mvmaus@mgh.harvard.edu; and John C. Byrd, The Ohio State University, CCC Building, 4th Floor, 410 W. 12th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu.
References
Author notes
J.A.F., K.A.B., J.C.B., and M.V.M. contributed equally to this study.

![Figure 3. Ibrutinib potentiates CTL019 cell expansion and engraftment, which correlates with clinical response. Ex vivo proliferation of total T cells during test expansion (A) and of CTL019 cells during clinical manufacturing (evaluated at day 9 after CD3/CD28-induced activation) (B) derived from patients with CLL and stratified by clinical response (n = 16 no response [NR], n = 7 partial response [PR], n = 7 CR) and by treatment (n = 3 pretreated with ibrutinib) compared with adults with ALL (n = 7). (C) In vivo engraftment of autologous CTL019 cells from the above CLL patients expressed as maximum expansion of adoptively transferred lymphocytes (copies of anti-CD19 CAR per microgram of genomic [g]DNA) in the first 90 days postinfusion. (D) Spearman ρ correlation shown between ex vivo expansion potential and in vivo proliferative capacity (maximum copies of anti-CD19 CAR per microgram of gDNA within 90 days after infusion) of adoptively transferred CTL019 cells in matched CLL patients (n = 30). *P < .05, **P < .01; error bars show mean ± standard error as determined by a 2-tailed Student t test. n.s., not statistically significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/9/10.1182_blood-2015-11-679134/4/m_1117f3.jpeg?Expires=1769080852&Signature=hImtQFKlanAm0iOxlSfJql8k1pDUiiRpu6y56DyU8kUjZQRQeIFAqNhMz4-4nmL0HjAIDk4VmgbZqyk5sLoFMjQoA3xpKUtY6TRoDUl9Umt8bMoahPDBzebMMIUe5G2NIB9lVTOrPXmsiMTtleSNgMs-2ZjgNTN7irHA0rBevsuUemfB7oUVPtdKQl37Cd697K4ZqxePbMU1dSH8XhCu5W7SA0bKYs~XNW2piMZFqDlgqbUftBmIT4GF4Sl3O75QT-XqFOByV-w8VCpQp40Pu8USbSzCHWySgTT1sFMlBIfuxgveOZ9CKpG9k3lMK42Hk4osVrriODX6rklYUQe~ng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Ibrutinib increases the engraftment and antitumor activity of CTL019 cells when administered concurrently. (A) Protocol schema depicting the engraftment of NOD scid gamma (NSG) mice with 106 luciferase-expressing Nalm-6 cells or 5 to 10 × 106 OSU-CLL cells. At day 7 or 9, animals (n = 5-12 per group) were randomized according to tumor burden to receive vehicle alone, 25 mg/kg per day of ibrutinib, or 106 CTL019 cells per mouse. Ibrutinib or empty vehicle was continuously administered for the entire duration of animal experiments. (B) Absolute numbers of adoptively transferred peripheral blood T cells were monitored weekly by retro-orbital bleeding and flow cytometric detection. (C) Bioluminescence images of 5 representative mice in the CTL019 and CTL019 + Ibr. treatment groups are shown at day 20 post–CAR T-cell infusion. (D) Peak bioluminescent (BLI) tumor burden depicted for each experimental group. (E) PD-1 expression on adoptively transferred CTL019 cells in the spleens of ibrutinib-naive or ibrutinib-treated mice at the time of euthanasia. (F) Overall survival of untreated mice (Nalm-6 alone [No Rx]) or animals treated with ibrutinib alone, CTL019 cells, or a combination of CTL019 and ibrutinib shown over time. Two separate experiments with similar results were performed. (G) In vivo expansion kinetics of CTL019 cells in mice with or without continuous ibrutinib treatment. Error bars show the standard error. (H) Survival of OSU-CLL-bearing NSG mice treated on day 7 after tumor injection with 106 CTL019 cells (n = 12 mice per group). Overall survival curves were plotted using the Kaplan-Meier method and compared using the log-rank (Mantel-Cox) test. Statistical significance between groups with differences in CTL019 expansion and tumor burden was determined using a 2-tailed Student t test. *P < .05, **P < .01, ***P < .001, ****P < .0001. CBG, click beetle green; n.s., not statistically significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/9/10.1182_blood-2015-11-679134/4/m_1117f6.jpeg?Expires=1769080852&Signature=rquIarn6i8~vnmr4baZJgG~tHJzoctrsEAbcYNjDNK2qgeCUdbDLxGqkaaVMT1R5ML4JA1mIUEUhuSUi1IOT9L~aKhr5spHf0wALf02JqRuBeVw9vpQrhnTpXfcMNh8KY40fqZXyDna~Ce4YwMpVtIZo05gC6Vr9IHoq1qKKyyMB8dzp8ehOFNq8XjXKlvJQSvrKVqUZkfGvu~G0RgwdsbS~xlBuS2CbjMdto84IRK0VDOw9Uhvr00wm1P9MzWwt8kNTbbZhcmXgLT~6W7l3kxgGdq6oYkJgqtjxcE9f8CZCvfzn26AFJH6kzPFP3pvZxidYiDWL58~JaJj2PQhSAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)