Key Points
In a multicenter, randomized phase 3 trial, MPR-R was not superior over MPT-T with respect to response rate, PFS, and OS.
Grade 3/4 hematologic toxicity requiring growth factor support occurred with MPR-R vs clinically significant neuropathy with MPT-T.
Abstract
The combination of melphalan, prednisone, and thalidomide (MPT) is considered standard therapy for newly diagnosed patients with multiple myeloma who are ineligible for stem cell transplantation. Long-term treatment with thalidomide is hampered by neurotoxicity. Melphalan, prednisone, and lenalidomide, followed by lenalidomide maintenance therapy, showed promising results without severe neuropathy emerging. We randomly assigned 668 patients between nine 4-week cycles of MPT followed by thalidomide maintenance until disease progression or unacceptable toxicity (MPT-T) and the same MP regimen with thalidomide being replaced by lenalidomide (MPR-R). This multicenter, open-label, randomized phase 3 trial was undertaken by Dutch-Belgium Cooperative Trial Group for Hematology Oncology and the Nordic Myeloma Study Group (the HOVON87/NMSG18 trial). The primary end point was progression-free survival (PFS). A total of 318 patients were randomly assigned to receive MPT-T, and 319 received MPR-R. After a median follow-up of 36 months, PFS with MPT-T was 20 months (95% confidence interval [CI], 18-23 months) vs 23 months (95% CI, 19-27 months) with MPR-R (hazard ratio, 0.87; 95% CI, 0.72-1.04; P = .12). Response rates were similar, with at least a very good partial response of 47% and 45%, respectively. Hematologic toxicity was more pronounced with MPR-R, especially grades 3 and 4 neutropenia: 64% vs 27%. Neuropathy of at least grade 3 was significantly higher in the MPT-T arm: 16% vs 2% in MPR-R, resulting in a significant shorter duration of maintenance therapy (5 vs 17 months in MPR-R), irrespective of age. MPR-R has no advantage over MPT-T concerning efficacy. The toxicity profile differed with clinically significant neuropathy during thalidomide maintenance vs myelosuppression with MPR.
Introduction
In many countries, standard therapy for patients with newly diagnosed multiple myeloma (NDMM) who are ineligible for autologous stem cell transplantation is melphalan and prednisone combined with a novel agent, currently either the immunomodulatory (IMiD) drug thalidomide (MPT) or the proteasome inhibitor bortezomib (VMP). A meta-analysis of 6 trials comparing MPT and MP showed that MPT significantly improved progression-free survival (PFS; median PFS, 20.3 vs 14.9 months) and overall survival (OS; median OS, 39.3 vs 32.9 months). The MPT regimens that were used in the 6 trials comparing MPT with MP were very heterogeneous with respect to the thalidomide scheme, ranging from during induction only to maintenance therapy until progression of the disease, with comparable duration of thalidomide therapy irrespective from the scheme. Therefore, in general, MPT followed by thalidomide therapy (MPT-T) regimens cannot be clearly discriminated from MPT regimens.1-7 Accordingly, the addition of bortezomib to MP (VMP) was found to result in a superior time to progression (TTP; median TTP, 24 vs 16.6 months with MP) and OS (median OS, 56.4 vs 43.1 months).8 However, up to 45% of patients had to discontinue therapy due to grade 3/4 toxicity. Severe neuropathy appeared to be an important cause of premature discontinuation of both thalidomide and bortezomib.8,9
In analogy to the MPT combination, the combination of MP with the second-generation IMiD, lenalidomide (MPR), has been explored.10 MPR followed by lenalidomide maintenance (MPR-R) resulted in a significant increase in median PFS compared with MP.11 The median PFS of 31 months seems superior to the outcome reached previously with MPT and VMP.1,12 However, this is probably explained by the continuous administration of lenalidomide, as 9 induction cycles of MPR only did not improve PFS compared with MP alone. Recently, a similar observation was made in the First trial, showing that continuous treatment with lenalidomide in combination with low-dose dexamethasone (Rd) resulted in a better PFS than treatment with Rd for 18 cycles only or MPT for 12 cycles only.13
We investigated whether lenalidomide instead of thalidomide, in combination with an MP backbone during induction, followed by maintenance therapy until disease progression (MPR-R vs MPT-T), improved the outcome of elderly MM patients not eligible for stem cell transplantation.
Patients and methods
Patients
Patients >65 years of age or patients ≤65 of age and not eligible for high-dose chemotherapy and peripheral stem cell transplantation with newly diagnosed symptomatic MM, measurable disease, and World Health Organization (WHO) performance status 0 to 3 (or 0-2 if ≥75 years) were eligible. Exclusion criteria were nonsecretory MM, known hypersensitivity to thalidomide, systemic amyloid light chain amyloidosis, neuropathy grade ≥2, severe cardiac dysfunction (New York Heart Association classification II-IV), severe pulmonary dysfunction, significant hepatic dysfunction (total bilirubin ≥30 µM or transaminases ≥3 times normal level) unless related to myeloma, creatinine clearance <30 mL/min, active uncontrolled infections, pretreatment with cytotoxic drug, IMiDs or proteasome inhibitors, HIV positivity, active malignancy during the last 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma, not able and/or not willing to use adequate contraception, and pregnancy. Radiotherapy or a short course of steroids (eg, 4-day treatment with dexamethasone 40 mg/day or equivalent) was allowed.
Trial design
This investigator-sponsored, open-label, randomized phase 3 trial was designed to show superiority of MPR-R over MPT-T and was performed by the Dutch-Belgium Cooperative Trial Group for Hematology Oncology (HOVON) and the Nordic Myeloma Study Group (NMSG) using a joint protocol, central randomization (randomly assigned 1:1 to MPT-T or MPR-R, stratified for hospital and International Staging System [ISS] stage [I vs II vs III]), data management, and analysis. This study was approved by the Ethics Committees of the participating sites. All patients gave written informed consent, and the trial was conducted according to the Declaration of Helsinki, ICH GCP Guidelines, the EU directive for Good Clinical Practice (2001/20/EG), and applicable regulatory requirements. This trial was registered at www.trialregister.nl as NTR1630 (EudraCT number 2007-004007-34).
Procedures
MPT induction was administered orally as 9 cycles of melphalan 0.18 mg/kg on days 1 to 4, prednisone 2 mg/kg on days 1 to 4, and thalidomide 200 mg/day until 4 weeks after the last cycle of MP. MPR induction included 9 cycles of oral treatment with melphalan 0.18 mg/kg on days 1 to 4, prednisone 2 mg/kg on days 1 to 4, and lenalidomide 10 mg on days 1 to 21, independent of age. Induction cycles were given every 28 days. Patients randomized to MPT-T received maintenance with oral thalidomide 100 mg daily, and patients randomized to MPR-R received maintenance with oral lenalidomide 10 mg on days 1 to 21 of every 28-day cycle until disease progression, independent of age. Dose adjustments are described in supplemental Tables 7 and 8, available on the Blood Web site, for neuropathy in specific. Thrombosis prophylaxis during induction therapy consisted of acetylsalicylic acid 75 or 80 mg or carbasalate calcium 100 mg daily. In patients with a history of venous thrombotic events, low-molecular-weight heparin was given instead. Bisphosphonate therapy and prophylactic antibiotics were given at the discretion of the physician. In case of an infectious event that required admission during induction therapy, prophylactic antibiotics (type of antibiotics according to local protocols: eg, quinolone, trimethoprim-sulfamethoxazole, or penicillin) were mandatory during the following courses of induction therapy. Patient and disease characteristics were registered at diagnosis. Interphase fluorescence in situ hybridization (FISH) on isolated CD138-positive plasma cells was performed at diagnosis according to the European Myeloma Network guidelines,14 investigating the presence of 14q32 abnormalities [t(4;14)(p16;q32) and t(14;16)(q32;q23)] and 17p13 loss (including the TP53 gene). In a subset of patients, the presence of 1q21 gain was determined.
Statistical analysis
For the sample size calculation, the expected median PFS in the MPT arm was 20 months, as obtained from the meta-analysis of 6 randomized clinical trials on MPT, in which 3 of 6 studies gave maintenance therapy with thalidomide.1 To detect a hazard ratio (HR) of 0.714, which corresponds to an increase of median PFS from 20 to 28 months in the MPR arm (2-sided significance level α = 0.05), with a power of 90% and assuming 4-year accrual and additional follow-up time of 1 year, 668 patients had to be randomized, and 377 events (ie, progressions or deaths) had to be observed before the final analysis could be performed. All analyses were performed according to intention to treat, restricted to eligible patients, and the primary analysis was done with a multivariate Cox regression including adjustment for ISS stage. Secondary end points included (improvement of) response, OS, and adverse events (AEs). Detailed statistical analyses and outcome parameters are provided in the supplemental Appendix.
Results
Patients
A total of 668 patients were included and randomized in the study from March 12, 2009 until October 19, 2012, of whom 31 were found not to be eligible (Figure 1). Of the 637 eligible patients, 318 patients were randomly assigned to MPT-T and 319 patients to MPR-R. The characteristics at baseline were well balanced (Table 1). FISH analysis on isolated plasma cells was performed in the majority of patients (73% in the MPT-T arm and 78% in the MPR-R arm).
CONSORT diagram. Reasons for noneligibility for MPT-T: no measurable disease (n = 7), no plasma cells and no plasmacytomas (n = 1), creatinine clearance <30 mL/min (n = 1), amyloidosis of the heart (n = 1), creatinine clearance <30 ml/min and amyloidosis (n = 1), polyneuropathy (PNP) grade 2 (n = 1), active malignancy last 5 years (n = 1), bladder cancer (n = 1), and registered twice by mistake (n = 1); for MPR-R: no measurable disease (n = 8), eligible for HDM and autoSCT (n = 1), angina pectoris grade 3 (n = 1), PNP grade 2 (n = 1), previous malignancy last 5 years (n = 2), pancreas carcinoma at entry (n = 1), presence of melanoma lentigo 2 months before randomization (n = 1), and registered twice by mistake (n = 1). Numbers reflect the number of patients starting with treatment in rectangle 1, proceeding to cycle 4 in rectangle 2, proceeding to cycle 7 in rectangle 4, and starting maintenance in rectangle 4. The number within the rectangles shown behind “cycles” indicate the maximum number of cycles that were given to these patients, except for the last cycle within a rectangle, as the majority of these patients proceed with therapy as can be deduced from the number of patients proceeding to either cycle 4 and 7.
CONSORT diagram. Reasons for noneligibility for MPT-T: no measurable disease (n = 7), no plasma cells and no plasmacytomas (n = 1), creatinine clearance <30 mL/min (n = 1), amyloidosis of the heart (n = 1), creatinine clearance <30 ml/min and amyloidosis (n = 1), polyneuropathy (PNP) grade 2 (n = 1), active malignancy last 5 years (n = 1), bladder cancer (n = 1), and registered twice by mistake (n = 1); for MPR-R: no measurable disease (n = 8), eligible for HDM and autoSCT (n = 1), angina pectoris grade 3 (n = 1), PNP grade 2 (n = 1), previous malignancy last 5 years (n = 2), pancreas carcinoma at entry (n = 1), presence of melanoma lentigo 2 months before randomization (n = 1), and registered twice by mistake (n = 1). Numbers reflect the number of patients starting with treatment in rectangle 1, proceeding to cycle 4 in rectangle 2, proceeding to cycle 7 in rectangle 4, and starting maintenance in rectangle 4. The number within the rectangles shown behind “cycles” indicate the maximum number of cycles that were given to these patients, except for the last cycle within a rectangle, as the majority of these patients proceed with therapy as can be deduced from the number of patients proceeding to either cycle 4 and 7.
Characteristics of patients at baseline
| . | Arm A: MPT-T . | Arm B: MPR-R . |
|---|---|---|
| Number | 318 | 319 |
| Median age, years (range) | 72 (60-91) | 73 (60-87) |
| Age ≥76 years, N (%) | 105 (33) | 110 (34) |
| Sex, N (%) | ||
| Male | 161 (51) | 185 (58) |
| Female | 157 (49) | 134 (42) |
| WHO performance status, N (%) | ||
| 0 | 104 (33) | 117 (37) |
| 1 | 152 (48) | 145 (45) |
| 2 | 43 (14) | 46 (14) |
| 3 | 6 (2) | 5 (2) |
| Unknown | 13 (4) | 6 (2) |
| M-protein subtype, N (%) | ||
| IgG | 202 (64) | 202 (63) |
| IgA | 87 (27) | 75 (24) |
| IgD | 5 (2) | 3 (1) |
| Light chain only | 22 (7) | 38 (12) |
| Unknown | 2 (1) | 1 (0) |
| ISS, N (%) | ||
| I | 75 (24) | 82 (26) |
| II | 153 (48) | 151 (47) |
| III | 83 (26) | 82 (26) |
| Unknown | 7 (2) | 4 (1) |
| Lactate dehydrogenase elevated, N (%) | 24 (8) | 33 (10) |
| Lytic bone disease (%) | 209 (66) | 219 (69) |
| FISH performed % | 231 (73) | 248 (78) |
| FISH abnormality present if performed, N (%) | ||
| 17p13 loss | 25/214 (12) | 19/221 (9) |
| t(4;14) | 21/224 (9) | 19/221 (8) |
| t(14;16) | 3/194 (2) | 10/214 (5) |
| 1q21 gain | 64/167 (38) | 67/188 (36) |
| . | Arm A: MPT-T . | Arm B: MPR-R . |
|---|---|---|
| Number | 318 | 319 |
| Median age, years (range) | 72 (60-91) | 73 (60-87) |
| Age ≥76 years, N (%) | 105 (33) | 110 (34) |
| Sex, N (%) | ||
| Male | 161 (51) | 185 (58) |
| Female | 157 (49) | 134 (42) |
| WHO performance status, N (%) | ||
| 0 | 104 (33) | 117 (37) |
| 1 | 152 (48) | 145 (45) |
| 2 | 43 (14) | 46 (14) |
| 3 | 6 (2) | 5 (2) |
| Unknown | 13 (4) | 6 (2) |
| M-protein subtype, N (%) | ||
| IgG | 202 (64) | 202 (63) |
| IgA | 87 (27) | 75 (24) |
| IgD | 5 (2) | 3 (1) |
| Light chain only | 22 (7) | 38 (12) |
| Unknown | 2 (1) | 1 (0) |
| ISS, N (%) | ||
| I | 75 (24) | 82 (26) |
| II | 153 (48) | 151 (47) |
| III | 83 (26) | 82 (26) |
| Unknown | 7 (2) | 4 (1) |
| Lactate dehydrogenase elevated, N (%) | 24 (8) | 33 (10) |
| Lytic bone disease (%) | 209 (66) | 219 (69) |
| FISH performed % | 231 (73) | 248 (78) |
| FISH abnormality present if performed, N (%) | ||
| 17p13 loss | 25/214 (12) | 19/221 (9) |
| t(4;14) | 21/224 (9) | 19/221 (8) |
| t(14;16) | 3/194 (2) | 10/214 (5) |
| 1q21 gain | 64/167 (38) | 67/188 (36) |
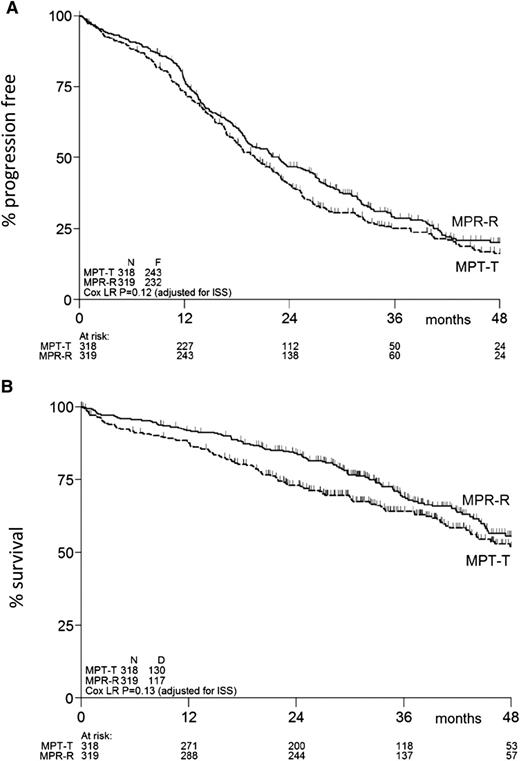
PFS and OS
At the time of this analysis, 475 events for PFS had been reported: 243 in the MPT-T arm vs 232 in the MPR-R arm. The flow of patients through the protocol is shown by a CONSORT diagram (Figure 1). The median PFS was similar in both arms: 20 months (95% confidence interval [CI], 18-23 months) with MPT-T vs 23 months (95% CI, 19-27 months) with MPR-R (HR, 0.87; 95% CI, 0.72-1.04; P = .12, adjusted for ISS; Figure 2A). A separate analysis of patients ≤75 and ≥76 years of age showed no effect of age on PFS (median, 20 [95% CI, 18-23] vs 20 months [95% CI, 15-23] with MPT-T and median 22 [95% CI, 19-27] vs 23 months [95% CI, 18-28] with MPR respectively). Overall, 51% (162 of 318) of patients started maintenance treatment with T and 59% (189 of 319) with R (P = .04). In the patients who started maintenance therapy, PFS from the start of maintenance therapy was not significantly different between both arms (median, PFS 17.4 months in the MPT-T arm vs 22.2 in the MPR-R arm; HR, 0.83; 95% CI, 0.63-1.08).
PFS and overall survival of eligible patients. Kaplan-Meier estimates of (A) PFS and (B) overall survival. N, number of patients; F, number of failures (ie, progression or death); D, number of deaths; ISS, International Staging System.
PFS and overall survival of eligible patients. Kaplan-Meier estimates of (A) PFS and (B) overall survival. N, number of patients; F, number of failures (ie, progression or death); D, number of deaths; ISS, International Staging System.
After a median FU of 36 months, 247 patients (39%) had died: 130 in the MPT-T arm vs 117 in the MPR-R arm. The OS at 2, 3, and 4 years in the MPT-T and MPR-R arms was 73% vs 84%, 64% vs 69%, and 52% vs 56%, respectively (HR, 0.82; 95% CI, 0.64-1.06; P = .13, adjusted for ISS; Figure 2B).
The HRs for PFS (HR, 0.84; 95% CI, 0.70-1.01; P = .06) and OS (HR, 0.79; 95% CI, 0.61-1.01; P = .06) showed a trend in favor of MPR-R in the multivariate analyses.
Subgroup analysis by age, ISS, and cytogenetic risk features showed that the HRs for PFS and OS (supplemental Figure 1) were consistent across subgroups, indicating no added value of lenalidomide over thalidomide. This was also observed in a nonprespecified analysis between patients with no more than a partial response (PR) and at least a very good PR (VGPR). Only in patients with ISS II was the HR for PFS 0.76 (95% CI, 0.59-0.98; P = .04), whereas in patients with t(4;14), MPR-R resulted in a superior OS (HR, 0.32; 95% CI, 0.11-0.87; P = .02).
The results of the planned univariate and multivariate analyses for the survival end points are shown in supplemental Tables 1 and 2. The results for multivariate analysis, restricted to patients with data available on 1q21 gain, 17p13 loss, and/or t(4;14), showed that each of these 3 abnormalities was associated with adverse PFS (P < .01), whereas 1q21 gain was also associated with significantly adverse OS. The PFS and OS for 1q21 gain was 17 (MPT-T) vs 19 (MPR-R) months and 39 (MPT-T) vs 50 (MPR-R) months, respectively. For del17p13, these numbers were 15 vs 15 months and 41 vs 35 months. For t(4:14), these numbers were 12 vs 14 months and 23 months vs not reached (P = .02 for OS). Elevated lactate dehydrogenase consistently was an adverse prognostic factor for both PFS (HR, 1.57; 95% CI, 1.14-2.16; P = .006) and OS (HR, 1.90; 95% CI, 1.28-2.83; P = .002). A higher WHO performance score, immunoglobulin (Ig)A M-protein, and higher ISS were associated with an adverse OS only.
Response rates
The overall response rate on protocol was similar in both arms: 81% with MPT-T and 84% with MPR-R. A high percentage of patients reached VGPR or better on protocol; 47% with MPT-T vs 45% with MPR-R, with complete response (CR) rates of 10% and 13%, respectively. The median time to response and the median time to maximum response were similar in both arms (Table 2; 2.7 vs 2.8 months and 4.4 vs 3.6 months, respectively). Overall response (data not shown) and depth of response increased over time (supplemental Figure 2). During maintenance treatment, 23% of patients showed a further upgrade in response in the MPT-T arm (including 9% from less than CR to CR and 13% from less than VGPR to VGPR) vs 18% in the MPR-R arm (including 8% from less than CR to CR and 8% from less than VGPR to VGPR).
Response rates and times to response on protocol
| Response rate, N (%) . | Arm A: MPT-T (N = 318) . | Arm B: MPR-R (N = 319) . |
|---|---|---|
| CR | 33 (10) | 40 (13) |
| VGPR | 117 (37) | 102 (32) |
| PR | 108 (34) | 125 (39) |
| ≥VGPR | 150 (47) | 142 (45) |
| Overall response on protocol (≥PR) | 258 (81) | 267 (84) |
| Median time to response (in months, range) | 2.7 (0.7-15.1) | 2.8 (0.7-33.1) |
| Median time to maximum response (in months, range) | 4.4 (0.9-45.7) | 3.6 (0.7-62.0) |
| Response rate, N (%) . | Arm A: MPT-T (N = 318) . | Arm B: MPR-R (N = 319) . |
|---|---|---|
| CR | 33 (10) | 40 (13) |
| VGPR | 117 (37) | 102 (32) |
| PR | 108 (34) | 125 (39) |
| ≥VGPR | 150 (47) | 142 (45) |
| Overall response on protocol (≥PR) | 258 (81) | 267 (84) |
| Median time to response (in months, range) | 2.7 (0.7-15.1) | 2.8 (0.7-33.1) |
| Median time to maximum response (in months, range) | 4.4 (0.9-45.7) | 3.6 (0.7-62.0) |
Toxicity
The proportion of treated patients with 1 or more grade 3 or 4 AEs during the full protocol treatment was 81% in the MPT-T group and 86% in the MPR-R group (P = .13). The number of serious adverse events was similar in both arms: 385 in the MPT-T arm and 383 in the MPR-R arm. The major reason for serious adverse events was hospitalization in 80% of patients in both arms. Eighty pecent resolved either without (65%) or with sequelae (15%). Complete safety data according to age are presented in supplemental Tables 3 (induction) and 4 (maintenance). The incidence of grade 3 or 4 hematologic toxicity was significantly higher in the MPR-R group compared with the MPT-T group (anemia, 14% vs 5%; thrombocytopenia, 30% vs 8%; neutropenia, 64% vs 27%; all P < .001). The use of granulocyte colony-stimulating factor was advised in case of grade 4 neutropenia or febrile neutropenia; 38% of patients were prescribed granulocyte colony-stimulating factor in the MPR-R group vs 16% in the MPT-T group (P < .001). The incidence of severe (grade 3 or 4) infections was similar in both groups (19% in MPR-R vs 21% in MPT-T; P = .69), as well as the incidence of febrile neutropenia (5% in MPR-R vs 3% in MPT-T; P = .23). The use of antibiotic prophylaxis was left at the discretion of the treating physician. In the MPR-R group, 22% used antibiotics vs 18% in the MPT-T group (P = .20). The incidence of venous thrombotic events grades 2 to 4 was 8% in both arms on protocol. Sixty-six percent of all patients received acetylsalicylic acid or carbasalate calcium only as thrombosis prophylaxis (70% in MPT-T vs 64% in MPR-R), 5% low-molecular-weight heparin (4% in MPT-T vs 5% in MPR-R), and 11% other (11% in both arms), and 18% of all patients used ≥2 different types (15% vs 21%). There was a significant difference in neuropathy (for induction and maintenance combined: at least grade 2 neuropathy, 44% in MPT-T vs 8% in MPR-R; at least grade 3 neuropathy, 16% in MPT-T vs 2% in MPR-R; both P < .001; results for induction and maintenance separately are shown in Table 3).
All grade neuropathy during induction and maintenance, in patients who started treatment
| Grade, N (%) . | MPT-T . | MPR-R . |
|---|---|---|
| Induction | ||
| N = 313 | N = 318 | |
| 0 | 159 (51) | 260 (82) |
| I | 76 (24) | 46 (14) |
| II | 57 (18) | 10 (3) |
| III | 21 (7) | 2 (1) |
| IV | — | — |
| Maintenance | ||
| N = 162 | N = 189 | |
| 0 | 69 (43) | 153 (81) |
| I | 14 (9) | 23 (12) |
| II | 50 (31) | 10 (5) |
| III | 29 (18) | 3 (2) |
| IV | — | — |
| Grade, N (%) . | MPT-T . | MPR-R . |
|---|---|---|
| Induction | ||
| N = 313 | N = 318 | |
| 0 | 159 (51) | 260 (82) |
| I | 76 (24) | 46 (14) |
| II | 57 (18) | 10 (3) |
| III | 21 (7) | 2 (1) |
| IV | — | — |
| Maintenance | ||
| N = 162 | N = 189 | |
| 0 | 69 (43) | 153 (81) |
| I | 14 (9) | 23 (12) |
| II | 50 (31) | 10 (5) |
| III | 29 (18) | 3 (2) |
| IV | — | — |
Discontinuation of induction therapy
Importantly, there was a high rate of discontinuation during induction therapy: 49% vs 41% in the MPT-T and MPR-R arms, respectively (Figure 1), mainly in patients >75 years of age (51% vs 32%; P = .04). Accordingly, the discontinuation rate due to toxicity was higher in patients >75 years (P = .005). Within treatment arms, this only appears to be significant for MPT (P = .036) and not for MPR (P = .057). There were 13 early deaths (Figure 1; within 3 cycles) during MPT induction vs 8 in the MPR induction. In the MPT arm, 25% was because of progressive disease, 45% because of infection, 25% because of cardiac events, and 5% unknown. In the MPR arm, it was 15% because of progressive disease and 85% because of infection. The demographics (similar parameters as described in Table 1) of patients who either reached or did not reach maintenance were comparable (data not shown).
Discontinuation and duration of maintenance therapy
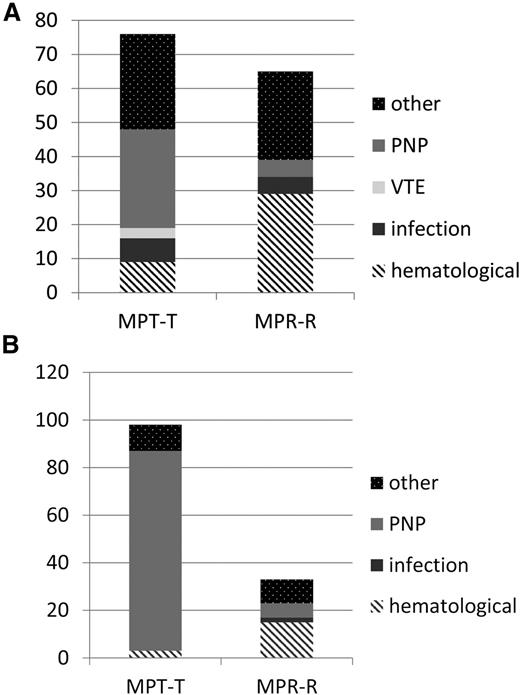
Significantly more patients had to discontinue maintenance therapy due to toxicity in the MPT-T arm vs the MPR-R arm (98 of 162 who started maintenance [60%] versus 33 of 189 who started maintenance [17%], respectively; P < .001). Age did not affect discontinuation rate. The development of neuropathy was the main reason for a significant higher discontinuation rate due to toxicity in the MPT-T group (Table 4; Figure 3; 84 of 98 patients who discontinued therapy due to neuropathy in MPT-T [87%] vs only 6 of 33 [3%] in MPR-R; P < .001). As a consequence, the median duration of maintenance therapy was significantly longer in the MPR-R group compared with the MPT-T group, irrespective of age (17 [range, 0-55] vs 5 months [range, 0-56] in patients ≤75 years, P < .001; and 15 [range, 1-54] vs 5 months [range, 0-44] in patients ≥76 years of age, P = .001; Table 4). In addition to discontinuation, dose reductions were recorded. The median relative dose intensity is given in supplemental Table 5. The cumulative dose of melphalan was found to be similar over the 2 arms: median, 400 vs 382 mg (P = .33).
Discontinuation rate during induction and maintenance in patients who started treatment
| . | MPT-T . | MPR-R . | ||
|---|---|---|---|---|
| ≤75 years (N = 209) . | ≥76 years (N = 104) . | ≤75 years (N = 209) . | ≥76 years (N = 109) . | |
| Induction | ||||
| Patients completing 6 induction cycles, N (%) | 162 (78) | 71 (68) | 160 (77) | 80 (73) |
| Patients reaching maintenance, N (%) | 121 (58) | 41 (39) | 124 (59) | 65 (60) |
| Discontinuation due to toxicity, N (%)* | 43 (21) | 33 (32) | 36 (17) | 29 (27) |
| Maintenance | ||||
| Number of patients starting maintenance | 121 | 41 | 124 | 65 |
| Number of patients discontinuing maintenance (%) | 109 (90) | 37 (90) | 87 (70) | 44 (68) |
| Discontinued due to toxicity, N (%)† | 73 (67) | 25 (68) | 20 (16) | 13 (20) |
| Median duration of maintenance therapy in months‡ | 5 | 5 | 17 | 15 |
| . | MPT-T . | MPR-R . | ||
|---|---|---|---|---|
| ≤75 years (N = 209) . | ≥76 years (N = 104) . | ≤75 years (N = 209) . | ≥76 years (N = 109) . | |
| Induction | ||||
| Patients completing 6 induction cycles, N (%) | 162 (78) | 71 (68) | 160 (77) | 80 (73) |
| Patients reaching maintenance, N (%) | 121 (58) | 41 (39) | 124 (59) | 65 (60) |
| Discontinuation due to toxicity, N (%)* | 43 (21) | 33 (32) | 36 (17) | 29 (27) |
| Maintenance | ||||
| Number of patients starting maintenance | 121 | 41 | 124 | 65 |
| Number of patients discontinuing maintenance (%) | 109 (90) | 37 (90) | 87 (70) | 44 (68) |
| Discontinued due to toxicity, N (%)† | 73 (67) | 25 (68) | 20 (16) | 13 (20) |
| Median duration of maintenance therapy in months‡ | 5 | 5 | 17 | 15 |
Significantly higher in all patients ≥76 vs ≤75 years (P = .005) and in the subgroup of patients in the MPT-T arm (P = .036) but not for MPR-R (P = .057).
Significantly higher in MPT-T vs MPR-R in all patients together, as well as in the subgroups of patients ≤75 and ≥76 years (P < .001).
Significantly shorter in MPT-T vs MPR-R in all patients together, as well as in the subgroups of patients ≤75 (P < .001) and ≥76 years (P = .001).
Toxicity-related discontinuation of therapy. (A) Reasons for discontinuation of therapy due to toxicity during induction. (B) Reasons for discontinuation of therapy due to toxicity during maintenance. y-axis absolute number of patients, reflecting the number of patients discontinuing therapy due to toxicity only. In MPT-T, there were 7 patients who went off protocol before maintenance due to other reasons: 1 for pulmonary hypertension, 1 for allergic reaction, 2 for toxicity, and 3 because of decision of physician. In the MPR-R arm, there were 5 patients who went off protocol before maintenance due to other reasons: 2 because of decision of physician and 3 for toxicity. In the thalidomide maintenance arm, 5 patients went off protocol due to other reasons: 2 for SPM, 1 died, and 2 because of refusal of patient and hematologic toxicity. In the lenalidomide maintenance arm, 18 patients went off protocol due to other reasons: 11 because of physician’s decision, mainly symptoms or side effects, 2 for dementia, 1 for renal failure, and 4 for SPM.
Toxicity-related discontinuation of therapy. (A) Reasons for discontinuation of therapy due to toxicity during induction. (B) Reasons for discontinuation of therapy due to toxicity during maintenance. y-axis absolute number of patients, reflecting the number of patients discontinuing therapy due to toxicity only. In MPT-T, there were 7 patients who went off protocol before maintenance due to other reasons: 1 for pulmonary hypertension, 1 for allergic reaction, 2 for toxicity, and 3 because of decision of physician. In the MPR-R arm, there were 5 patients who went off protocol before maintenance due to other reasons: 2 because of decision of physician and 3 for toxicity. In the thalidomide maintenance arm, 5 patients went off protocol due to other reasons: 2 for SPM, 1 died, and 2 because of refusal of patient and hematologic toxicity. In the lenalidomide maintenance arm, 18 patients went off protocol due to other reasons: 11 because of physician’s decision, mainly symptoms or side effects, 2 for dementia, 1 for renal failure, and 4 for SPM.
Given the similar efficacy of both treatment arms with a pronounced difference in duration of maintenance therapy, it was investigated whether a different selection of patients in the MPT-T arm vs the MPR-R arm reached the maintenance phase. From 367 patients, all cytogenetic abnormalities at diagnosis were known, allowing definition of the cytogenetic risk [17p13 loss, t(4;14), 1q21 gain, or a combination, defined as high risk, or neither of these 3, defined as standard risk]. Of the standard-risk patients, 49 of 87 (56%) of patients reached maintenance therapy in the MPT-T arm and 63 of 106 (59%) in the MPR-R arm. However, of the high-risk patients, only 39 of 88 (44%) started maintenance therapy in the MPT-T arm vs 54 of 86 (63%) in the MPR-R arm.
Second primary malignancies
The total number of patients with 1 or more second primary malignancies (SPMs) was similar in both groups (supplemental Table 6): 28 and 39 in MPT-T and MPR-R, respectively, (P = .37). Invasive second primary cancers (excluding non-melanoma skin cancer) were reported in 23 (7%) patients who received MPT-T vs in 19 (6%) who received MPR-R, resulting in an incidence rate of 2.9 and 2.1 per 100 patient-years, respectively (P = .34). The number of non-melanoma skin cancer was significantly higher in the MPR-R arm: 20 vs 5 in MPT-T (P = .006).
Discussion
In this phase 3 trial in elderly patients with NDMM not eligible for autologous stem cell transplantation, there was no difference in response, PFS, and OS between MPR-R and MPT-T. We hypothesized a superior efficacy of MPR-R because of the median PFS of 31 months with MPR-R previously described by Palumbo et al.4 Although patients were older in our study (≥76 years; 34% vs 24% in the MM015 study) and in the MM015 study the discontinuation rate due to toxicity was higher in patients ≥76 years, this will probably not be the explanation for the difference in PFS. We performed a separate analysis on PFS in patients ≤75 years and patients ≥76 years, showing no difference (22 and 23 months, respectively, vs 31 and 19 months in the MM015 study). Accordingly, we found that the discontinuation rate due to toxicity and the median relative dose intensity of melphalan in MPR-R–treated patients was similar over the age groups, as opposed to the MM015 study. Importantly, a recent Eastern Cooperative Oncology Group (ECOG) study, comparing similar regimens, also found equal efficacy of MPR-R and MPT-T, with a PFS in the MPR-R arm even being slightly worse compared with what we observed, which might be explained by a lower dose of melphalan in the MPR arm: 5 vs 9 mg/m2 in the MPT-T arm.15 Therefore, the only explanation for the superior PFS in the MM015 trial is the difference in follow-up: 30 vs 36 months in our trial, with a considerable number of patients not having reached the median PFS yet. This is supported by the fact that at 12 and 20 months, the percentage of patients without progression is rather comparable between the MM015 and our trial: 80% vs 78% and 58% vs 54%, respectively.
In contrast to comparable efficacy of MPR-R and MPT-T, there was a pronounced difference in toxicity, especially neuropathy. The significantly higher incidence of grade 3 and 4 neuropathy was the main reason for the high discontinuation rate of nearly 70% during thalidomide maintenance, with a median duration of therapy of only 5 months. In contrast, lenalidomide maintenance was feasible, irrespective of age, and was supported by a similar duration of maintenance treatment of 17 and 15 months in patients ≤75 and >75 years, respectively. Similar to our study, in the ECOG study, less toxicity was reported in the MPR-R arm. This translated into a better quality of life.15 In contrast to the ECOG trial, in which less than grade 4 hematologic toxicity was not required to be reported, in our study, hematologic toxicity could be determined. Grade 3/4 hematologic toxicity was significantly higher in the MPR-R arm, requiring growth factor support in 38% of patients. Given the fact that the majority of early deaths in the MPR-R arm was due to infections, antibiotic prophylaxis and more stringent use of growth factors is probably important.
One might have expected that the significantly longer duration of lenalidomide maintenance vs thalidomide maintenance would have translated into a superior PFS. It might be that the dose of lenalidomide is too low, which is supported by the fact that there is a fall in the PFS at 9 months, just after the start of maintenance therapy, whereas such a fall has not been observed in the First trial, with a continuing therapy of 25 mg lenalidomide. Moreover, in our study, it was found that the percentage of patients with standard-risk cytogenetics that had reached the maintenance phase was similar in the MPT-T and MPR-R arms, whereas less patients with high-risk cytogenetics reached the maintenance phase in the MPT-T arm. Therefore, at the start of maintenance therapy, there is selection of good-risk patients in the MPT-T arm, which did not occur in the MPR-R arm. Thalidomide maintenance therapy was indeed found to improve outcome in patients with a standard-risk cytogenetic profile previously, whereas in high-risk patients, even a negative impact of thalidomide maintenance therapy was observed.16,17 However, an alternative explanation might also be that the optimal duration of maintenance therapy differs between thalidomide and lenalidomide.
The incidence of SPM was similar in the 2 arms, with a higher incidence of non-melanoma skin cancers in the MPR-R arm only. The incidence of SPM in our study is similar to that described in the MM015 study and the First trial. However, the incidence of hematologic malignancies we observed in the MPR-R arm is higher compared with Rd in the First trial. This is consistent with the hypothesis that the increase in second hematologic primary malignancies is related to prior or concurrent use of oral melphalan.18,19
Because of the similar efficacy of both regimens, but a pronounced difference in neuropathy, in our opinion, MPR-R is the preferred regimen over MPT-T. However, on a global perspective, taking accessibility and costs of novel agents and the required growth factors using MPR-R into account, a role for MPT-T, especially in good-risk patients, remains, provided that close monitoring of the development of neuropathy and early discontinuation of thalidomide is secured. In contrast to MPR-R in our HOVON/NMSG trial, not showing a PFS or OS advantage over MPT, the Intergroupe Francophone du Myélome First trial showed that Rd resulted in a superior PFS and OS over 12 cycles of MPT. This cross-trial comparison supports the use of Rd in clinical practice, although a head-to-head comparison is lacking. Our observation that in patients with t(4;14), MPR-R results in a superior OS over MPT-T is interesting, as in the First trial, no such benefit of Rd was observed in high-risk patients [del17p, t(4;14) and/or t(14;16)]. However, numbers are small, and in the First trial, separate data in t(4;14) are lacking. Phase 3 randomized clinical trials in elderly NDMM patients, comparing IMiD-based regimens with either proteasome inhibitors and monoclonal antibodies or combinations of these 3 classes of drugs, are currently lacking, but will shape the treatment landscape in coming years, with a special emphasis on the unmet clinical need of high-risk patients.
In conclusion, MPR-R has no advantage over MPT-T with respect to response rate, PFS, and OS. However, the use of thalidomide as maintenance therapy was associated with a high rate of clinically significant neuropathy and is therefore not preferred for maintenance strategies. In contrast, the hematologic toxicity profile of MPR-R did require growth factor support but did not translate in a higher clinical infection rate, and therefore is also manageable in patients >75 years of age.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the local and central data managers for collecting and verifying patient data. The FISH data were reviewed by M. Stevens-Kroef, D. Olde Weghuis, and A. Buijs. The Data Safety Monitoring Board for this trial consisted of A. Palumbo, Italy; J. Bladé, Spain; and K. Wheatley, United Kingdom. We also thank the HOVON Data Center for the continuous support in the trial conduct.
This study was supported by Dutch Cancer Society grant 2008-4246, the Norwegian Cancer Society, and Celgene.
Authorship
Contribution: S.Z. designed research, performed research, collected data, analyzed and interpreted data, and wrote the manuscript; B.v.d.H. designed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; U.-H.M. designed research and collected data; M.S. designed research and collected data; G.M.J.B. collected data; M.-D.L. collected data; H.V.-W. collected data; M.H. collected data; A.W.G.v.d.V. collected data; W.D. collected data; A.G. collected data; J.L.L.M.C. collected data; T.P. collected data; S.K.K. collected data; B.C.T. collected data; D.L.S. collected data; R.E.B. collected data; M.W. collected data; M.(R.)B.L.L. collected data; H.A.M.S. collected data; E.H. collected data; K.G.v.d.H. collected data; M.F.D. collected data; E.(V.)J.M.M. collected data; N.W.C.J.v.d.D. collected data; M.J.P.L.S.-K. collected data; P.S. designed research, collected data, and analyzed and interpreted data; and A.W. designed research, collected data, and analyzed and interpreted data.
Conflict-of-interest disclosure: S.Z. has a consulting/advisory role for Celgene, Takeda, and Janssen and receives research funding Celgene, Takeda, and Janssen; U.-H.M. has received honoraria from Celgene, Janssen, Amgen, and Mundipharma; G.M.J.B. has a consulting/advisory role for Celgene and Amgen, receives research funding from Celgene, and holds patents/intellectual property at the University Medical Center Maastricht; M.-D.L. has a consulting/advisory role for Celgene and Amgen; J.L.L.M.C. has a consulting/advisory role for Sanofi-Aventis Netherlands B.V.; T.P. has received honoraria from Janssen, Genmab, and Celgene, has a consulting/advisory role for Genmab and Janssen, and receives research funding from Janssen; E.H. has a consulting/advisory role for Amgen, Celgene, Janssen, and Novartis; N.W.C.J.v.d.D. has a consulting/advisory role for Amgen, Celgene, and Janssen, is on the speaker’s bureau for Amgen, Celgene, and Janssen, and has received research funding from Amgen, Celgene, and Janssen; P.S. has received honoraria from Janssen, Celgene, Onyx, and Novartis and research funding from Janssen, Celgene, and Onyx; and A.W. has received honoraria from Novartis and Amgen, has a consultancy or advisory role for Celgene, and is on the speaker’s bureau for Novartis. All other authors declare no competing financial interests.
Correspondence: Sonja Zweegman, Department of Hematology, 2PK brug 014, VU University Medical Center, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: s.zweegman@vumc.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal