Key Points
Triplet lenalidomide-based regimens did not induce any advantage over doublet lenalidomide-based regimens in elderly myeloma patients.
Abstract
Lenalidomide-dexamethasone improved outcome in newly diagnosed elderly multiple myeloma patients. We randomly assigned 662 patients who were age ≥65 years or transplantation-ineligible to receive induction with melphalan-prednisone-lenalidomide (MPR) or cyclophosphamide-prednisone-lenalidomide (CPR) or lenalidomide plus low-dose dexamethasone (Rd). The primary end point was progression-free survival (PFS) in triplet (MPR and CPR) vs doublet (Rd) lenalidomide-containing regimens. After a median follow-up of 39 months, the median PFS was 22 months for the triplet combinations and 21 months for the doublet (P = .284). The median overall survival (OS) was not reached in either arms, and the 4-year OS was 67% for the triplet and 58% for the doublet arms (P = .709). By considering the 3 treatment arms separately, no difference in outcome was detected among MPR, CPR, and Rd. The most common grade ≥3 toxicity was neutropenia: 64% in MPR, 29% in CPR, and 25% in Rd patients (P < .0001). Grade ≥3 nonhematologic toxicities were similar among arms and were mainly infections (6.5% to 11%), constitutional (3.5% to 9.5%), and cardiac (4.5% to 6%), with no difference among the arms. In conclusion, in the overall population, the alkylator-containing triplets MPR and CPR were not superior to the alkylator-free doublet Rd, which was associated with lower toxicity. This study was registered at www.clinicaltrials.gov as #NCT01093196.
Introduction
Multiple myeloma (MM) is the second most frequent hematologic cancer, with a median age at diagnosis of ∼70 years.1 MM is still an incurable disease, but novel agents, such as the proteasome inhibitor bortezomib and immunomodulatory drugs thalidomide and lenalidomide have considerably improved progression-free survival (PFS) and overall survival (OS).2-6 In Europe, melphalan-prednisone-thalidomide (MPT) and melphalan-prednisone-bortezomib (VMP) are considered the standards of care for MM patients older than age 65 years or not eligible for autologous stem cell transplantation.2,3,7-13 Recently, two large phase 3 trials have shown the superiority of lenalidomide-containing regimens over the standard treatments approved for elderly patients with newly diagnosed MM.14,15 The MM-015 trial showed that combined melphalan-prednisone-lenalidomide (MPR) followed by maintenance with lenalidomide (MPR-R) significantly prolonged PFS (31 months) compared with melphalan-prednisone (13 months; P < .001) or MPR without maintenance (14 months; P < .001). The major benefit was observed in patients age 65 to 75 years (P = .001 for treatment-by-age interaction).14 The FIRST trial showed that lenalidomide plus low-dose dexamethasone (Rd) given until disease progression was associated with a significant improvement in PFS (25.5 months) when compared with melphalan-prednisone-thalidomide (21.2 months) or Rd (20.7 months) for a fixed period of 18 months (hazard ratio [HR] for the risk of progression or death was 0.72 for continuous Rd vs MPT and 0.70 for continuous Rd vs 18 months of Rd; P < .001 for both comparisons).The advantage of Rd given continuously or for a fixed period was evident in patients older than or younger than age 75 years.15
To date, a formal comparison between an alkylator-containing triplet regimen vs an alkylator-free doublet regimen, both including lenalidomide, has not yet been performed. In this phase 3 trial, we compared a triplet lenalidomide-containing regimen (MPR or cyclophosphamide-prednisone-lenalidomide [CPR]) with a doublet lenalidomide-containing regimen (Rd) to evaluate which was the best drug to combine with lenalidomide (alkylating agents or steroids). The primary end point was PFS with the triplet vs the doublet lenalidomide-containing regimens.
Patients and methods
Study patients
Patients with newly diagnosed MM who were ineligible for high-dose therapy plus stem cell transplantation because of age (≥65 years) or coexisting comorbidities could be enrolled. Inclusion criteria were measurable disease and Karnofsky performance status ≥60%. Patients agreed to use contraception, and women of childbearing age had a pregnancy test before enrollment. Exclusion criteria included renal impairment (creatinine level <30 mL/min), uncontrolled or severe cardiovascular disease, and other malignancies within the past 3 years. The study was approved by the institutional review board at each of the participating centers. All patients gave written informed consent before entering the study, which was performed in accordance with the Declaration of Helsinki.
Study design and intervention
This was a multicenter randomized (1:1:1) phase 3 clinical trial that involved 58 centers in Italy and 9 centers in the Czech Republic. The primary end point was PFS; secondary end points included response rate, time to the first evidence of response, OS, and incidence of any grade 3 or higher adverse events. Per protocol, patients were stratified by age (75 years or younger vs older than 75 years). On the basis of the recent International Myeloma Working Group geriatric score that stratifies patients according to their frailty status (fit, intermediate fitness, and frail),16 a post hoc analysis not prespecified in the original protocol was conducted that included age (80 years or younger vs older than 80 years), comorbidities (according to Charlson score), and cognitive and/or physical status (according to the Activities of Daily Living and the Instrumental Activities of Daily Living scores). The definitions of fit, intermediate-fitness, and frail patients based on age, Charlson score, the Activities of Daily Living and the Instrumental Activities of Daily Living scores are summarized in the supplemental Data available on the Blood Web site.
Patients enrolled were randomly allocated to receive induction treatment with nine 28-day cycles of MPR (n = 217) or CPR (n = 220) or Rd (n = 217). First-line dose reductions of dexamethasone, melphalan and cyclophosphamide were performed according to the patient’s age. MPR patients received lenalidomide 10 mg per day for 21 days, oral melphalan 0.18 mg/kg for 4 days in patients age 65 to 75 years or 0.13 mg/kg in those older than age 75 years, and prednisone 1.5 mg/kg for 4 days. CPR patients received lenalidomide 10 mg per day for 21 days, oral cyclophosphamide 50 mg every other day for 28 days in patients age 65 to 75 years or 50 mg every other day for 21 days in those older than age 75 years, and prednisone 25 mg every other day. Rd patients received lenalidomide 25 mg per day for 21 days, dexamethasone 40 mg on days 1, 8, 15, and 22 in patients age 65 to 75 years or 20 mg in those older than age 75 years.
After induction, patients were randomly assigned to receive maintenance treatment with lenalidomide alone at 10 mg on days 1 to 21 every 28 days or in combination with prednisone at 25 mg every other day continuously. After the inclusion of the first 120 patients, the protocol was amended to increment the dose of lenalidomide and cyclophosphamide in patients age 65 to 75 years in the CPR arm as a result of negligible toxicities compared with the 2 other treatment arms. The CPR induction schedule was changed to lenalidomide 25 mg per day for 21 days and oral cyclophosphamide 50 mg per day for 21 days. All new patients randomly assigned to the CPR arm received treatment according to the new regimen after the amendment was approved. Treatment was withheld on withdrawal of the patient’s consent, disease progression, or the occurrence of any grade 4 hematologic adverse events or grade 3 to 4 nonhematologic adverse event; less serious toxicities were managed through established dose-modification guidelines. Antithrombotic prophylaxis was mandatory: aspirin or low-molecular-weight heparin or warfarin were permitted at physician’s discretion.
Assessments of end point
The primary end point was PFS in patients treated with triplet compared with those who received a doublet combination. PFS was calculated from the time of induction randomization until the date of progression, relapse, death as a result of any cause, or the date the patient was last known to be in remission. OS was a secondary end point in triplet vs doublet regimens and was calculated from the time of induction randomization until the date of death as a result of any cause or the date the patient was last known to be alive. Efficacy and safety assessments were performed every 4 weeks until relapse, or until evidence of disease progression, or when clinically indicated. Evaluation of the response to the treatments was performed according to the International Response Criteria for Multiple Myeloma.17 Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Statistical analysis
The study was designed as a 2 × 2 factorial trial, with two main comparisons of PFS: between induction regimens (triplet vs doublet) and between maintenance treatments (lenalidomide-prednisone vs lenalidomide alone). The design of the study was to show superiority of a 3-drug regimen over a 2-drug regimen. A sample size of 640 patients (430 in the MPR plus CPR arms vs 210 in the Rd arm) was determined to provide a power of 80% to detect a PFS HR ≤0.75 comparing patients in the MPR and CPR arms with those in the Rd arm by using a log-rank test with a two-sided α of .05. An interim safety analysis was planned when ∼85 patients had received at least 1 treatment. Patients were analyzed on an intention-to-treat basis for all time-to-event end points. Times of observation were censored on November 1, 2014. Response rates and safety were analyzed in patients who received at least 1 dose of study drugs. Response rates and the incidence of any adverse event were compared by using the χ2 test or Fisher’s exact test when appropriate. Survival data were analyzed by using the Kaplan-Meier method, and treatment arms were compared with the log-rank test. Time to event was expressed as median with interquartile range. The Cox proportional hazard model was used to estimate HR values and the 95% confidence intervals (CIs) for the intention-to-treat population.
Results
Between August 2009 and September 2012, a total of 662 patients were enrolled. Eight patients were excluded from randomization for screening failure (Figure 1). Six hundred fifty-four patients were randomly assigned to receive induction with MPR (n = 217) or CPR (n = 220) or Rd (n = 217). Patient baseline and demographic characteristics were well balanced among the 3 arms (Table 1). Median age was 74 years in the MPR arm, 73 years in the CPR arm, and 73 years in the Rd arm. About 25% of patients were classified as frail. At the time of analysis, all patients had completed the assigned induction treatment, and 402 patients were randomly allocated to maintenance treatment. The median duration of treatment was 18 months (range, 1 to 62 months).
Patient demographic and baseline characteristics
| Characteristic . | MPR (n = 218) No. (%) . | CPR (n = 222) No. (%) . | Rd (n = 222) No. (%) . |
|---|---|---|---|
| Age, y | 63-91 | 63-87 | 50-89 |
| Median | 74 | 73 | 73 |
| >75 | 80 (37) | 73 (33) | 77 (35) |
| Male sex | 108 (50) | 106 (48) | 108 (49) |
| Karnofsky score | 60-100 | 60-100 | 60-100 |
| Median | 80 | 90 | 90 |
| <80 | 52 (24) | 44 (20) | 44 (20) |
| Fitness | |||
| Fit | 89 (41) | 98 (44) | 98 (45) |
| Intermediate fitness | 79 (36) | 70 (32) | 57 (26) |
| Frail | 49 (23) | 54 (24) | 65 (28) |
| Data missing | 1 (1) | 0 | 2 (1) |
| Creatinine clearance (mL/min) | 30-168 | 30-152 | 30-150 |
| Median | 70 | 67 | 65 |
| International Staging System score | |||
| I | 61 (28) | 59 (27) | 62 (28) |
| II | 97 (45) | 103 (46) | 99 (45) |
| III | 59 (27) | 60 (27) | 60 (27) |
| Missing data | 1 (0.5) | 0 | 1 (0.5) |
| Cytogenetic abnormalities at FISH | |||
| Data available | 163 (75) | 177 (80) | 185 (83) |
| Data missing | 55 (25) | 45 (20) | 37 (17) |
| High risk* | 38 (17) | 48 (22) | 47 (25) |
| Characteristic . | MPR (n = 218) No. (%) . | CPR (n = 222) No. (%) . | Rd (n = 222) No. (%) . |
|---|---|---|---|
| Age, y | 63-91 | 63-87 | 50-89 |
| Median | 74 | 73 | 73 |
| >75 | 80 (37) | 73 (33) | 77 (35) |
| Male sex | 108 (50) | 106 (48) | 108 (49) |
| Karnofsky score | 60-100 | 60-100 | 60-100 |
| Median | 80 | 90 | 90 |
| <80 | 52 (24) | 44 (20) | 44 (20) |
| Fitness | |||
| Fit | 89 (41) | 98 (44) | 98 (45) |
| Intermediate fitness | 79 (36) | 70 (32) | 57 (26) |
| Frail | 49 (23) | 54 (24) | 65 (28) |
| Data missing | 1 (1) | 0 | 2 (1) |
| Creatinine clearance (mL/min) | 30-168 | 30-152 | 30-150 |
| Median | 70 | 67 | 65 |
| International Staging System score | |||
| I | 61 (28) | 59 (27) | 62 (28) |
| II | 97 (45) | 103 (46) | 99 (45) |
| III | 59 (27) | 60 (27) | 60 (27) |
| Missing data | 1 (0.5) | 0 | 1 (0.5) |
| Cytogenetic abnormalities at FISH | |||
| Data available | 163 (75) | 177 (80) | 185 (83) |
| Data missing | 55 (25) | 45 (20) | 37 (17) |
| High risk* | 38 (17) | 48 (22) | 47 (25) |
FISH, fluorescent in situ hybridization.
At least one among deletion17p (del17) or translocation (4;14) [t(4;14)] or translocation (14;16) [t(14;16)].
Efficacy
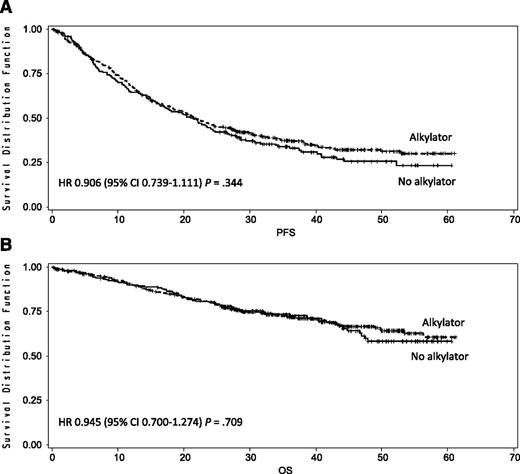
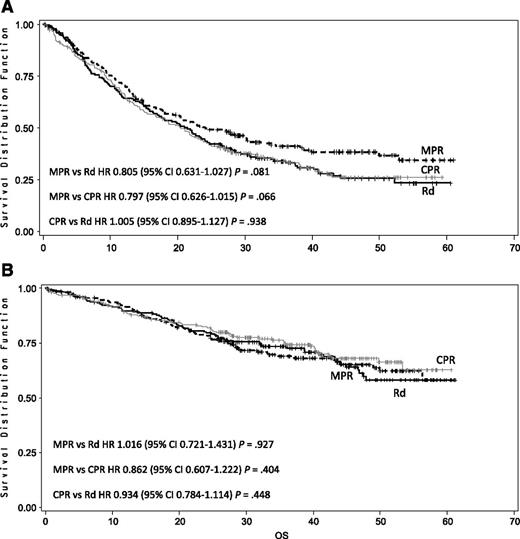
After a median follow-up of 39 months, the median PFS was 22 months with triplet and 21 months with doublet regimens (HR, 0.906; 95% CI, 0.739-1.111; P = .344; Figure 2A). The median OS was not reached; the 4-year OS was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709; Figure 2B). By comparing the 3 arms separately, the median PFS was 24 months in the MPR, 20 months in the CPR, and 21 months in the Rd arm (Figure 3A). The 4-year OS was 65% with MPR, 68% with CPR, and 58% with Rd (Figure 3B). After nine induction cycles, the overall response rate (at least partial response) was similar in the 3 arms: 71% with MPR, 68% with CPR, and 74% with Rd (Table 2).
Kaplan-Meier estimates of PFS and OS in doublet regimens with no alkylating agents vs triplet regimens with alkylating agents after a median follow-up of 39 months. (A) Median PFS was 21 months in doublet regimens with no alkylating agents vs 22 months in triplet regimens with alkylating agents. (B) Four-year OS was 58% in the doublet regimens with no alkylating agents vs 67% in the triplet regimens with alkylating agents.
Kaplan-Meier estimates of PFS and OS in doublet regimens with no alkylating agents vs triplet regimens with alkylating agents after a median follow-up of 39 months. (A) Median PFS was 21 months in doublet regimens with no alkylating agents vs 22 months in triplet regimens with alkylating agents. (B) Four-year OS was 58% in the doublet regimens with no alkylating agents vs 67% in the triplet regimens with alkylating agents.
Kaplan-Meier estimates of PFS and OS according to treatment arm. (A) Median PFS was 21 months with Rd, 24 months with MPR, and 20 months with CPR. (B) Four-year OS was 58% with Rd, 65%with MPR, and 68% with CPR.
Kaplan-Meier estimates of PFS and OS according to treatment arm. (A) Median PFS was 21 months with Rd, 24 months with MPR, and 20 months with CPR. (B) Four-year OS was 58% with Rd, 65%with MPR, and 68% with CPR.
Response rates
| Response . | MPR (n = 211) No. (%) . | CPR (n = 220) No. (%) . | Rd (n = 212) No. (%) . |
|---|---|---|---|
| Overall response rate | 150 (71) | 150 (68) | 157 (74) |
| Complete response | 7 (3) | 1 (0.5) | 6 (3) |
| Very good partial response | 48 (23) | 44 (20) | 65 (31) |
| Partial response | 95 (45) | 105 (48) | 86 (41) |
| Stable disease | 51 (24) | 62 (28) | 49 (23) |
| Not evaluable* | 8 (4) | 3 (1) | 5 (2) |
| Progressive disease | 2 (1) | 5 (2) | 1 (0.5) |
| Median time to response, mo | 2 | 2 | 1.8 |
| Response . | MPR (n = 211) No. (%) . | CPR (n = 220) No. (%) . | Rd (n = 212) No. (%) . |
|---|---|---|---|
| Overall response rate | 150 (71) | 150 (68) | 157 (74) |
| Complete response | 7 (3) | 1 (0.5) | 6 (3) |
| Very good partial response | 48 (23) | 44 (20) | 65 (31) |
| Partial response | 95 (45) | 105 (48) | 86 (41) |
| Stable disease | 51 (24) | 62 (28) | 49 (23) |
| Not evaluable* | 8 (4) | 3 (1) | 5 (2) |
| Progressive disease | 2 (1) | 5 (2) | 1 (0.5) |
| Median time to response, mo | 2 | 2 | 1.8 |
Patients not evaluable for not completing first induction cycle: Rd: 1 sudden death, 1 death not specified, 1 heart failure, 1 medical decision, 1 lost at follow-up; MPR: 4 adverse events (2 fever of unknown origin, 1 not specified, 1 diarrhea and renal failure), 2 lost at follow-up, 1 death as a result of pneumonia, and 1 sudden death; CPR: 1 withdrawal of consent, 1 death as a result of sepsis, and 1 death as a result of atrial fibrillation.
A post hoc analysis according to patient frailty was performed (supplemental Data). By the primary comparison, in fit patients, the median PFS was 23 months with the triplet regimens and 22 months with the doublet regimen, and the 4-year OS was 77% and 57%, respectively. In intermediate-fitness patients, the median PFS was 22 months with the triplet and 20 months with the doublet regimen, and the 4-year OS was 67% and 72%, respectively. In frail patients, the median PFS was 18 months with the triplet and 22 months with the doublet regimen, and the 4-year OS was 44% and 50%, respectively.
When the 3 arms were compared separately, in fit patients, the median PFS was 30 months in the MPR, 22 months in the CPR, and 22 months in the Rd arm (MPR vs Rd: HR, 0.671; 95% CI, 0.461-0.976; P = .037; supplemental Figure panel B). The 4-year OS was 77% in both MPR and CPR arms and 57% in the Rd arm. In intermediate-fitness patients, the median PFS was 19 months in the MPR, 23 months in the CPR, and 20 months in the Rd arm. The 4-year OS was 61% in the MPR arm and 72% in both the CPR and Rd arms. In frail patients, the median PFS was 23 months in the MPR, 14 months in the CPR, and 22 months in the Rd arm. The 4-year OS was 43% in the MPR, 45% in the CPR, and 50% in the Rd arm.
Safety
During the induction treatment, the most frequent grade ≥3 toxicities were hematologic. At least 1 grade ≥3 hematologic adverse event was reported in 68% of MPR, 32% of CPR, and 29% of Rd patients (P < .0001; Table 3). Neutropenic fever occurred in 5 MPR (3%), 4 CPR (2%), and 2 Rd patients (1%). Per protocol, granulocyte colony-stimulating factor was administered in case of febrile neutropenia and grade 3 to 4 neutropenia. In this study, 57% of MPR, 23% of CPR, and 20% of Rd patients (P < .0001) received granulocyte colony-stimulating factor, reducing the duration of neutropenia and the risk of infections. The rate of at least 1 grade ≥3 nonhematologic adverse event did not exceed 31% in the 3 arms. The most frequent grade ≥3 nonhematologic toxicities were infections (11% with MPR, 6.5% with CPR, and 9% with Rd), constitutional events (9.5% with MPR, 3.5% with CPR, and 5% with Rd), and cardiac toxicities (4.5% with MPR, 6% with CPR, and 6% with Rd), and no significant differences were detected among the 3 arms (Table 3). A very low incidence of thromboembolic events was recorded: 3% in MPR, 5% in CPR, and 2% in Rd patients. Among the 643 evaluable patients, 203 patients (32%) received low-molecular-weight heparin, 300 (47%) aspirin, 87 (14%) both, 16 (2%) warfarin, and 37 (5%) did not receive any prophylaxis. Grade ≥3 peripheral neuropathy was not significant in any of the arms. Thirteen cases of second primary malignancies (SPMs) were recorded: 7 (3%) in the MPR, 3 (1%) in the CPR, and 3 (1%) in Rd arm. Of these, 5 SPMs occurred during induction: 3 (1.5%) in the MPR and 2 (1%) in the CPR arm. All SPMs were solid except for 1 case of acute lymphoblastic leukemia in the CPR arm. Median time to SPM occurrence was 15 months (range, 5-36 months). The rate of discontinuation as a result of adverse events was similar in the three arms: 37 (18%) of 211 in the MPR, 33 (15%) of 220 in the CPR, and 30 (14%) of 212 in the Rd arm. Lenalidomide was reduced in 45 MPR (21%), 40 CPR (18%), and 34 Rd patients (16%), with no significant differences among the 3 arms. During the induction phase, 27 deaths not related to the progression of disease occurred: 8 in the MPR (4%), 9 in the CPR (5%), and 10 in the Rd arm (4%). Nineteen deaths were related to the treatment: 5 in the MPR (1 sudden death, 3 infections, and 1 stroke), 8 in the CPR (1 pulmonary embolism, 5 cardiologic toxicities, 1 stroke, and 1 infection), and 6 in the Rd (1 sudden death, 1 decline of general condition, 1 stroke, and 3 cardiologic toxicities) arm.
Grade ≥3 adverse events during induction treatment
| Grade ≥3 adverse event . | MPR (n = 211) No. (%) . | CPR (n = 220) No. (%) . | Rd (n = 212) No. (%) . |
|---|---|---|---|
| Hematologic | |||
| At least one event | 143 (68) | 71 (32) | 61 (29) |
| Anemia | 32 (15) | 14 (6) | 9 (4) |
| Neutropenia* | 136 (64) | 63 (29) | 52 (25) |
| Thrombocytopenia | 37 (18) | 19 (9) | 15 (7) |
| Nonhematologic | |||
| At least one event | 66 (31) | 66 (30) | 63 (30) |
| Cardiologic | 9 (4.5) | 11 (6) | 13 (6) |
| Arrythmia | 2 (1) | 2(1) | 3 (1.5) |
| Acute myocardial infarction | 1 (0.5) | 4(2) | 4 (2) |
| Heart failure | 2(1) | 3(1.5) | 4 (2) |
| Other | 4(2) | 2(1) | 2 (1) |
| Vascular | 7 (3.5) | 12 (5) | 7 (3) |
| Deep vein thrombosis/thromboembolism | 6 (3) | 10 (5) | 5 (2) |
| Stroke | 1 (0.5) | 2 (1) | 2 (1) |
| Constitutional | 19 (9.5) | 7 (3.5) | 11 (5) |
| Fever | 10 (5) | 2 (1) | 3 (1.5) |
| Fatigue | 6 (3) | 4(2) | 5(2) |
| Other | 3 (1.5) | 1(0.5) | 3(1.5) |
| Dermatologic | 9 (5) | 17 (8) | 11 (5) |
| Infection | 23 (11) | 16 (6.5) | 20 (9) |
| Pneumonia | 2 (1) | 6(2.5) | 4(2) |
| Bronchitis | 1 (0.5) | 0 | 3 (1.5) |
| Sepsis | 2 (1) | 2 (1) | 2 (1) |
| Enteritis | 0 | 2 (1) | 2 (1) |
| Febrile neutropenia | 8 (4) | 4 (2) | 3 (1.5) |
| Viral reactivation | 6 (2) | 0 | 1 (0.5) |
| Other/not specified | 4 (1) | 2 (1) | 5 (1) |
| Peripheral neurolopathy | 6 (3) | 6 (3) | 5 (2) |
| Second primary malignancies | 3 (1.5) | 2 (1) | 0 |
| Hematologic | 0 | 1 (0.5) | 0 |
| Solid | 3 (1.5) | 1 (0.5) | 0 |
| Discontinuation due to adverse events | 37 (18) | 33 (15) | 30 (14) |
| Grade ≥3 adverse event . | MPR (n = 211) No. (%) . | CPR (n = 220) No. (%) . | Rd (n = 212) No. (%) . |
|---|---|---|---|
| Hematologic | |||
| At least one event | 143 (68) | 71 (32) | 61 (29) |
| Anemia | 32 (15) | 14 (6) | 9 (4) |
| Neutropenia* | 136 (64) | 63 (29) | 52 (25) |
| Thrombocytopenia | 37 (18) | 19 (9) | 15 (7) |
| Nonhematologic | |||
| At least one event | 66 (31) | 66 (30) | 63 (30) |
| Cardiologic | 9 (4.5) | 11 (6) | 13 (6) |
| Arrythmia | 2 (1) | 2(1) | 3 (1.5) |
| Acute myocardial infarction | 1 (0.5) | 4(2) | 4 (2) |
| Heart failure | 2(1) | 3(1.5) | 4 (2) |
| Other | 4(2) | 2(1) | 2 (1) |
| Vascular | 7 (3.5) | 12 (5) | 7 (3) |
| Deep vein thrombosis/thromboembolism | 6 (3) | 10 (5) | 5 (2) |
| Stroke | 1 (0.5) | 2 (1) | 2 (1) |
| Constitutional | 19 (9.5) | 7 (3.5) | 11 (5) |
| Fever | 10 (5) | 2 (1) | 3 (1.5) |
| Fatigue | 6 (3) | 4(2) | 5(2) |
| Other | 3 (1.5) | 1(0.5) | 3(1.5) |
| Dermatologic | 9 (5) | 17 (8) | 11 (5) |
| Infection | 23 (11) | 16 (6.5) | 20 (9) |
| Pneumonia | 2 (1) | 6(2.5) | 4(2) |
| Bronchitis | 1 (0.5) | 0 | 3 (1.5) |
| Sepsis | 2 (1) | 2 (1) | 2 (1) |
| Enteritis | 0 | 2 (1) | 2 (1) |
| Febrile neutropenia | 8 (4) | 4 (2) | 3 (1.5) |
| Viral reactivation | 6 (2) | 0 | 1 (0.5) |
| Other/not specified | 4 (1) | 2 (1) | 5 (1) |
| Peripheral neurolopathy | 6 (3) | 6 (3) | 5 (2) |
| Second primary malignancies | 3 (1.5) | 2 (1) | 0 |
| Hematologic | 0 | 1 (0.5) | 0 |
| Solid | 3 (1.5) | 1 (0.5) | 0 |
| Discontinuation due to adverse events | 37 (18) | 33 (15) | 30 (14) |
Administration of granulocyte colony-stimulating factor: Rd, 43 (20%); MPR, 120 (57%); CPR, 51 (23%).
In a post hoc analysis, the incidence of at least 1 hematologic adverse event in fit patients was 75% for those who received MPR, 34% for CPR, and 29% for Rd (P = .0001 for both MPR vs Rd and MPR vs CPR). The rate of nonhematologic adverse events was 25% in MPR, 22% in CPR, and 27% in Rd patients. The rate of discontinuation as a result of adverse events was 13% in patients treated with MPR, 8% in patients treated with CPR, and 10% in those treated with Rd. Three fit patients died as a result of treatment-related toxicity (1 per arm). In intermediate-fitness patients, the incidence of hematologic toxicity was 61% in MPR, 33% in CPR, and 25% in Rd (MPR vs Rd and MPR vs CPR, P = .0001). At least 1 nonhematologic adverse event occurred in 29% of MPR, 32% of CPR, and 26% of Rd patients. An increased rate of discontinuation as a result of toxicities was detected independently of treatment randomization: 20% with MPR, 13% with CPR, and 18% with Rd. Two intermediate-fitness patients in the MPR and 2 in the Rd arm died due to treatment-related toxicities. In frail patients, the incidence of at least 1 hematologic adverse event was 75% with MPR, 28% with CPR, and 3% with Rd (P = .0001 for both MPR vs Rd and MPR vs CPR). The incidence of nonhematologic toxicities was 47% in MPR, 42% in CPR, and 38% in Rd patients. Frail patients had the highest rate of discontinuation as a result of adverse events, and this was more evident in the alkylator-containing regimens: 23% with MPR, 30% with CPR, and 18% with Rd. A higher number of frail patients died due to toxicity: 2 in the MPR (2 infections), 7 in the CPR (5 cardiologic events, 1 infection, and 1 stroke), and 3 in the Rd (3 cardiologic events) arm.
Discussion
This is the first randomized phase 3 trial that compared two alkylator-containing triplet regimens (MPR and CPR) with an alkylator-free doublet regimen (Rd) in patients with MM who were ineligible for stem cell transplantation. After a median follow-up of 39 months, no difference in PFS was noticed between triplet and doublet regimens; thus the hypothesis of the trial has not been confirmed. By analyzing the 3 arms separately, we found that the addition of an alkylating agent did not lead to any advantage in terms of response and outcome.
In our study, the median PFS with Rd was slightly shorter than that reported in the FIRST study (25.5 months with continuous Rd and 20.7 months with Rd for 18 months).15 In addition, our response rate was comparable to the response rate reported in the FIRST trial, in which the overall response rate with Rd was 73% to 75%. However, we reported a complete response rate of 3% with Rd, which is lower than the 15% reported in the FIRST trial. Of note, the median duration of continuous Rd in that trial was 18.4 months, whereas in our study, Rd was administered for only 9 months as induction treatment followed by maintenance, which included lenalidomide at a lower dose. Maintenance therapy might have had an impact on PFS, but the current follow-up does not allow us to draw definitive conclusions. A future analysis with a longer follow-up is planned to better evaluate the impact of maintenance therapy. The more intense regimens of the FIRST study induced an increase in the extrahematologic toxicity. This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability.
In our trial, the major safety concern was the higher hematologic toxicity reported with MPR compared with that for CPR and Rd (P < .0001). Nonhematologic adverse events were comparable in the 3 arms, with an incidence not higher than 10%. In the FIRST study, a higher incidence of infections (29% in continuous Rd, 22% in Rd for 18 months, and 17% in MPT) and cardiac events (12% in continuous Rd, 7% in Rd for 18 months, and 9% in MPT) was reported.15 This difference could be the result of longer administration of dexamethasone in the FIRST trial compared with our study. A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.
In our trial, the incidence of SPM (2%) was not higher than the rates reported in other studies with lenalidomide-containing regimens. Of note, the incidence was higher with the alkylator-containing regimens, with 3 cases of SPM reported in the Rd arm versus 10 cases in the MPR and CPR arms. This is in line with a previous meta-analysis that demonstrated an increased risk of SPM with lenalidomide in combination with melphalan.18
Cyclophosphamide was shown to be less toxic and was associated with a lower risk of SPM than melphalan, and thus may be considered an alternative.18,19 Nevertheless, we found no particular advantage in terms of efficacy with CPR over the 2-drug Rd regimen. Despite the amendment, we might have adopted a dose of cyclophosphamide that was too low in our study. Because of this limitation, no definitive conclusions can be drawn regarding CPR. To date, in the elderly setting, 1 phase 2 trial has evaluated cyclophosphamide 300 mg/m2 in combination with carfilzomib and dexamethasone with positive results.19 This may provide the rationale for also testing this dose in lenalidomide-containing regimens.
Determining treatment doses based only on age could be a limit. Therefore, we conducted a post hoc analysis and classified patients as fit, intermediate fitness, and frail.16 With all the limitations of a post hoc analysis, we found a PFS advantage with MPR compared with Rd (HR, 0.671; P = .037) and CPR in fit patients. Intermediate-fitness and frail patients did not benefit from the addition of an alkylating agent. Hematologic toxicities were similar in fit, intermediate-fitness, and frail patients within each treatment arm. MPR was confirmed to be the combination with the highest incidence of hematologic adverse events, independently of the patients’ frailty status. Conversely, frailty influenced the risk of nonhematologic toxicities, discontinuation, and treatment-related deaths. In frail patients in the MPR arm, infection was the only cause of death, thus reflecting the marked immune depression with this combination; in frail patients in the CPR and Rd arms, cardiovascular toxicity was the major cause of death, and this is in line with the toxicity profile of the 2 combinations. Nevertheless, caution is necessary when interpreting these data because the frailty analysis was not prespecified; thus, no definitive conclusions can be drawn. Future studies that include frailty evaluation may validate our results. In real life, a simple geriatric evaluation in the outpatient setting can be performed and may be a valuable tool to guide clinicians in the treatment decision process.
In conclusion, this trial showed that in real-life elderly myeloma patients, the alkylator-containing MPR or CPR triplet regimens were not superior to the alkylator-free Rd doublet. This is in line with the registrational FIRST study, in which Rd was demonstrated to be effective for all elderly patients.15
In this era of novel effective drugs, new attractive therapeutic options are now available, and Rd should be optimized through the addition of novel agents. Recently, the addition of carfilzomib to Rd was shown to be effective in the relapsed setting,20 and results of a phase 1/2 trial demonstrated that this combination is well tolerated and is also effective in newly diagnosed patients.21 Monoclonal antibodies such as elotuzumab were shown to be effective in combination with Rd in the relapsed setting, and further investigation in the newly diagnosed setting is needed.22 In the future, trials will confirm the role of novel agents in this setting, and these agents may increase the treatment armamentarium against myeloma.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients who participated in the study, nurses Silvia Boscolo and Concetta Calicchio, data managers Antonella Fiorillo and Elena Tigano, and editorial assistant Giorgio Schirripa.
Authorship
Contribution: V. Magarotto, S.B., G.C., M.B., and A.P. designed the study and supervised its conduct and the data analysis; V. Magarotto, S.B., M.O., G.B., F.P., R.M., A.P.F., L.D.P., G.P., S.G., C.M., N.G., A.B., C.C., S.P., V. Maisnar, M.R., R.Z., T.G., A.L., A.M.L., V. Montefusco, and R.H. recruited patients in the source studies and provided relevant data; V. Magarotto collected and assembled the data; G.C. performed the statistical analysis; V. Magarotto, S.B., and A.P. analyzed and interpreted the data; V. Magarotto and A.P. drafted the initial manuscript; and all authors were given unrestricted access to the data, critically reviewed the manuscript drafts, approved the final version, and made the decision to submit the manuscript for publication.
Conflict-of-interest disclosure: S.B., M.O., and S.G. received honoraria from Celgene; F.P. served on the advisory boards for Mundipharma, Bristol-Myers Squibb, Janssen, and Merck Sharp & Dohme (MSD) and received honoraria from MSD, Janssen, and Celgene; N.G. received research support from Celgene and Janssen; V. Maisnar received consultancy fees from Celgene and Janssen and honoraria from Amgen; R.Z. served on the advisory boards for Celgene and Janssen-Cilag; T.G. received research support from Celgene; R.H. received consultancy fees from Celgene and Janssen and honoraria from Amgen; M.B. received research support and consultancy fees from Celgene and served on their scientific advisory board; and A.P. received consultancy fees and honoraria from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Antonio Palumbo, University of Torino, Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Via Genova 3, 10126 Torino, Italy; e-mail: appalumbo@yahoo.com.