Key Points
Patients with breast cancer have a risk of VTE equivalent to 6% a year while undergoing chemotherapy and in the month after treatment.
Tamoxifen is associated with a risk of VTE equivalent to 2% a year, which is 4 times higher than the risk before starting therapy.
Abstract
Patients with breast cancer are at increased risk of venous thromboembolism (VTE), particularly in the peridiagnosis period. However, no previous epidemiologic studies have investigated the relative impact of breast cancer treatments in a time-dependent manner. We aimed to determine the impact of breast cancer stage, biology, and treatment on the absolute and relative risks of VTE by using several recently linked data sources from England. Our cohort comprised 13 202 patients with breast cancer from the Clinical Practice Research Datalink (linked to Hospital Episode Statistics and Cancer Registry data) diagnosed between 1997 and 2006 with follow-up continuing to the end of 2010. Cox regression analysis was performed to determine which demographic, treatment-related, and biological factors independently affected VTE risk. Women had an annual VTE incidence of 6% while receiving chemotherapy which was 10.8-fold higher (95% confidence interval [CI], 8.2-14.4; absolute rate [AR], 59.6 per 1000 person-years) than that in women who did not receive chemotherapy. After surgery, the risk was significantly increased in the first month (hazard ratio [HR], 2.2; 95% CI, 1.4-3.4; AR, 23.5; reference group, no surgery), but the risk was not increased after the first month. Risk of VTE was noticeably higher in the 3 months after initiation of tamoxifen compared with the risk before therapy (HR, 5.5; 95% CI, 2.3-12.7; AR, 24.1); however, initiating therapy with aromatase inhibitors was not associated with VTE (HR, 0.8; 95% CI, 0.5-1.4; AR, 28.3). In conclusion, women receiving chemotherapy for breast cancer have a clinically important risk of VTE, whereas an increased risk of VTE immediately after endocrine therapy is restricted to tamoxifen.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 953.
Disclosures
Thomas L. Ortel, Associate Editor, served as an advisor or consultant for Instrumentation Laboratory and received grants for clinical research from Instrumentation Laboratory; Eisai; Pfizer; and GlaxoSmithKline. The authors and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Identify the effect of chemotherapy for breast cancer on the risk for venous thromboembolism, based on an English cohort study.
Determine the effect of breast cancer surgery on the risk for venous thromboembolism.
Distinguish the effect of tamoxifen and aromatase inhibitors for breast cancer treatment on the risk for venous thromboembolism.
Release date: February 18, 2016; Expiration date: February 18, 2017
Introduction
Women with breast cancer have a three- to fourfold increased risk of venous thromboembolism (VTE) compared with women of an equivalent age without cancer.1,2 Because breast cancer is the most common cancer worldwide,3 this equates to a substantial impact of breast cancer-related VTE for patients and medical resources. Breast cancer–associated VTE accounts for approximately 17% of cancer-related VTEs presenting to anticoagulation clinics.4 It is also associated with increased disease recurrence,5 but more importantly, reduced survival5,6 among patients for whom prognosis is otherwise comparatively good.
Previous cohort studies have identified several risk factors for VTE in breast cancer patients including metastatic disease,2,7,8 chemotherapy,2,8,9 and tamoxifen treatment.10 A recent systematic review demonstrated that on average, breast cancer patients selected because they had either metastatic disease or were undergoing surgery or chemotherapy had a 10-fold increase in VTE risk compared with the breast cancer population as a whole.11 Although these studies go some way toward highlighting which groups are at highest risk of VTE, none have comprehensively assessed the relative importance of cancer treatments and biology in influencing VTE risk by using prospectively gathered data.
Identifying combinations of between and within patient factors would allow us to develop algorithms to guide thromboprophylaxis in the setting of breast cancer. Guidelines issued by the National Comprehensive Cancer Network emphasize that general use of thromboprophylaxis in patients receiving chemotherapy remains controversial and that more data are needed before risk-adjusted thromboprophylaxis can be routinely introduced in clinical practice.12 Guidelines from the American College of Chest Physicians also advise against routine prophylaxis for cancer outpatients unless they have additional risk factors, including previous thrombosis, immobilization, hormonal therapy, and angiogenesis inhibitors, a recommendation based on low-grade evidence.13 In both instances, there is limited guidance for specific cancer types (including breast) for which the influence of other risk factors could vary substantially. Identifying the patients most at risk is problematic partly because of the absence of precise and accurate data on absolute risks of VTE during specific times of the disease course. We have addressed this by ascertaining the incidence rate of VTE in relation to tumor biology (cancer grade and stage), intrinsic patient factors (age, body mass index [BMI] and comorbidity), and cancer treatments (surgery, chemotherapy, and endocrine therapy) by using 4 recently linked health care databases from the United Kingdom.
Methods
Patients and data sources
We used data from 4 linked health care sources. The Clinical Practice Research Datalink (CPRD) is a prospectively gathered, anonymized primary care database that uses data from more than 600 general practices (GPs) in the United Kingdom from 1987 onward. It provides all recorded primary care data on patients, including clinical diagnoses and prescriptions, and is known to be broadly representative of the population of the United Kingdom in terms of age, sex, and socioeconomic and geographic distribution.14 Hospital episode statistics (HES) is a secondary care database containing data for all hospitalizations in England, including primary and secondary discharge diagnoses and inpatient procedures. Information on cancer diagnoses was obtained from the National Cancer Intelligence Network, which processes data supplied by all regional cancer registries in the United Kingdom.15 Two related but separate databases make up the cancer registry data; the Merged Cancer Registry (from English registries only) and the Office of National Statistics (ONS) minimum cancer data set. Detailed information on specific data items collected by cancer registries in England for breast cancer patients over the period of this study (1997 to 2006) along with the completeness of recording for each of the TNM components we used to define cancer stage can be found elsewhere.16 Finally, we used death certificate data from the ONS, which provides information on dates and underlying causes of death. This analysis is based on patients from approximately 50% of CPRD practices in England for whom data linked to the HES, National Cancer Intelligence Network, and ONS data sources are available from April 1997 onward. The study received approval from the CPRD Independent Scientific Advisory Committee (protocol No. 10_091).
We selected all patients who had a first breast cancer diagnosis (International Classification of Diseases Revision 10 [ICD-10], code C50) between April 1, 1997, and December 31, 2006. Patients were observed until they developed a VTE event, died, left a participating GP, or December 31, 2010, whichever was earliest. The earliest recorded date in the cancer registry data was used to determine date of cancer diagnosis. Patients were excluded if they were male, younger than 18 years of age, not in a linked GP, diagnosed with breast cancer outside of the CPRD and HES registration periods, diagnosed in the first year of registration at a participating practice, or had a VTE prior to first cancer diagnosis.
Risk factors
Cancer stage and grade at diagnosis were obtained from the cancer registry database. If stage was known, we classified it as either “local disease” (confined to the breast), “regional disease” (axillary lymph node involvement), or “distant metastases” (any evidence of distant metastases). Conversion from TNM staging into these summary stages was performed according to the algorithm designed by Ording et al.17 Cancer treatments were defined on the basis of an associated Office of Population Censuses and Surveys Classification of Surgical Operations and Procedures (4th revision) code for chemotherapy and surgery by using the hospital admissions data. Surgery codes were specific to procedures used in the treatment of breast cancer. For chemotherapy, events were frequently recorded as a series of day case or outpatient procedures and were considered part of the same course of treatment when occurring within 28 days of each other. Broad Office of Population Censuses and Surveys Classification of Surgical Operations and Procedures (4th revision) codes for chemotherapy were used, which limited our potential to study specific chemotherapy regimens. We distinguished endocrine treatment with tamoxifen from the newer aromatase inhibitors (AIs), both of which were obtained from the GP prescription record. We assumed that women without endocrine prescriptions had estrogen receptor–negative breast cancer. BMI was determined from GP records on the basis of the most recent recording prior to cancer diagnosis, and GP records were also used to calculate an individual comorbidity score (Charlson Comorbidity Index, but ignoring the breast cancer diagnosis which was universal in our cohort) for each patient (coded as 0, 1-3, ≥4).18
Outcome
Primary care (CPRD) medical diagnoses were recorded by using Read codes, and secondary care (HES) diagnoses were recorded using ICD-10 codes. A VTE event was confirmed when a medical code for VTE (ICD-10; I26, I80-I82) in either CPRD or HES or both was supported by an anticoagulant prescription or a medical code providing evidence of anticoagulation if either one was recorded between 15 days before and 90 days after the VTE event date, or if death occurred within 30 days of the event. In addition, an underlying cause of death as a result of VTE was included as evidence of a valid VTE event. Only the first VTE event after the diagnosis of cancer was considered because follow-up would cease at this time. Therefore, all subsequent VTE events were ignored. This algorithm for defining VTE has been previously validated by using primary care data alone, although this validation was carried out in women who developed VTE after using oral contraceptives rather than in women with breast cancer.19
Statistical methods
Absolute rates (ARs) of VTE (per 1000 person-years) were calculated on the basis of all risk factors listed above by dividing the number of people with VTE by the person-time at risk. To establish the independent effects of these risk factors, Cox regression was used to obtain adjusted hazard ratios (HRs). BMI, comorbidity, cancer stage, and cancer grade were all treated as time-independent covariates, whereas the effects of cancer treatments were allowed to vary by time. Each variable was examined on its own within the breast cancer cohort and then adjusted for all other variables in the model regardless of their own significance. Adjusted HRs are presented in “Results.”
For surgery and chemotherapy, we then assessed the absolute VTE rate before treatment, during treatment, and in monthly periods after treatment. For endocrine treatment, we assessed the risk before therapy, in the 3-month interval after the initial prescription, and subsequent follow-up time. These periods were selected to account for the fact that endocrine therapy is usually administered continuously for a minimum of 5 years. For all treatment variables, patients who did not undergo the treatment composed the reference category. Date of cancer diagnosis was always used to denote study entry, but calendar time was used as the timescale for our analysis so that both the unadjusted and adjusted HRs would account for any temporal confounding.
In subsequent analyses, we looked at the joint relationship between surgery and chemotherapy to account for the fact that chemotherapy often takes place when women are recovering from surgery. Assuming a long period of excess VTE risk after the completion of surgery or chemotherapy (3 months) allowed us to explore ARs in the absence of potential carryover effects from the other treatment. We also looked at the interaction between cancer stage and the effects of surgery and chemotherapy. There were missing data in our cohort for cancer stage, grade, and BMI. We accounted for these missing data by creating a category comprising women with missing data. When analyses were repeated using multiple imputation by chained equations to impute missing values, results were very similar. All analyses used STATA version 13 (STATA, College Station, TX).
Results
Patient characteristics
A total of 13 202 women were diagnosed with breast cancer between 1997 and 2006 (Table 1). Women were a median age of 62 years at cancer diagnosis (interquartile range [IQR], 52 to 74 years). A total of 4% had metastatic disease at diagnosis and 38% had local disease (36% had stage unknown). Seventy-seven percent of the sample underwent surgery at some point after cancer diagnosis, 21% underwent chemotherapy, and 82% were prescribed endocrine therapy. First surgery occurred on average 17 days (IQR, 0-31 days) after cancer diagnosis. Among women who had primary surgery for breast cancer, chemotherapy began an average of 52 days (IQR, 33-105 days) after surgery. The median follow-up time from diagnosis was 5.3 years (IQR, 2.8-8.0 years). VTE occurred in 611 patients among 72 596 person-years of follow-up, which corresponds to a rate of 8.4 per 1000 person-years (95% confidence interval [CI], 7.8-9.1). This rate was 3.5 times (95% CI, 3.2-3.9) higher than in age-matched controls, as shown in our previous report on this cohort.1 The rate of VTE in breast cancer patients increased over the time period of the study from 5.4 (95% CI, 3.6 to 8.2) per 1000 person-years for women with breast cancer diagnosed in 1997 to 10.5 (95% CI, 8.5 to 13.2) per 1000 person-years for 2005. Of the women who experienced VTE events, 273 developed pulmonary embolism either with or without deep vein thrombosis (DVT), 314 developed DVT alone, and 24 had other thrombosis events. In most instances, the type of DVT was not specified (n = 175), but when it was specified, 13 of 139 events were upper extremity events.
Characteristics of patients with breast cancer
| Characteristic . | Patients (n = 13 202) . | Patients with VTE (n = 611) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Cancer stage | ||||
| Local disease | 5 037 | 38.2 | 214 | 4.3 |
| Regional disease | 2 961 | 22.4 | 161 | 5.4 |
| Distant metastases | 470 | 3.6 | 21 | 4.5 |
| Unknown | 4 734 | 35.9 | 215 | 4.5 |
| Grade | ||||
| Well differentiated | 1 681 | 12.7 | 71 | 4.2 |
| Moderately differentiated | 4 232 | 32.1 | 196 | 4.6 |
| Poorly differentiated | 3 024 | 22.9 | 166 | 5.5 |
| Unknown | 4 265 | 32.3 | 178 | 4.2 |
| Age (years) | ||||
| <40 | 715 | 5.4 | 21 | 2.9 |
| 40-49 | 2 030 | 15.4 | 63 | 3.1 |
| 50-59 | 3 262 | 24.7 | 108 | 3.3 |
| 60-69 | 2 973 | 22.5 | 179 | 6.0 |
| 70-79 | 2 407 | 18.2 | 158 | 6.6 |
| ≥80 | 1 815 | 13.8 | 82 | 4.5 |
| Comorbidity | ||||
| 0 | 6 987 | 52.9 | 295 | 4.2 |
| 1 to 3 | 5 860 | 44.4 | 293 | 5.0 |
| ≥4 | 355 | 2.7 | 23 | 6.5 |
| Body mass index (kg/m2) | ||||
| Underweight (<18.5) | 192 | 1.5 | 4 | 2.1 |
| Ideal (18.5-24.9) | 3 099 | 23.5 | 93 | 3.0 |
| Overweight (25.0-29.9) | 2 520 | 19.1 | 148 | 5.9 |
| Obese (30.0-39.9) | 1 119 | 8.5 | 73 | 6.5 |
| Morbidly obese (≥40.0) | 440 | 3.3 | 38 | 8.6 |
| Missing | 5 832 | 44.2 | 255 | 4.4 |
| Surgery | ||||
| No | 3 093 | 23.4 | 104 | 3.4 |
| Yes | 10 109 | 76.6 | 507 | 5.0 |
| Chemotherapy | ||||
| No | 10 429 | 79.0 | 422 | 4.1 |
| Yes | 2 773 | 21.0 | 189 | 6.8 |
| Endocrine therapy | ||||
| No | 2 323 | 17.6 | 87 | 3.7 |
| Yes | 10 879 | 82.4 | 524 | 4.8 |
| Characteristic . | Patients (n = 13 202) . | Patients with VTE (n = 611) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Cancer stage | ||||
| Local disease | 5 037 | 38.2 | 214 | 4.3 |
| Regional disease | 2 961 | 22.4 | 161 | 5.4 |
| Distant metastases | 470 | 3.6 | 21 | 4.5 |
| Unknown | 4 734 | 35.9 | 215 | 4.5 |
| Grade | ||||
| Well differentiated | 1 681 | 12.7 | 71 | 4.2 |
| Moderately differentiated | 4 232 | 32.1 | 196 | 4.6 |
| Poorly differentiated | 3 024 | 22.9 | 166 | 5.5 |
| Unknown | 4 265 | 32.3 | 178 | 4.2 |
| Age (years) | ||||
| <40 | 715 | 5.4 | 21 | 2.9 |
| 40-49 | 2 030 | 15.4 | 63 | 3.1 |
| 50-59 | 3 262 | 24.7 | 108 | 3.3 |
| 60-69 | 2 973 | 22.5 | 179 | 6.0 |
| 70-79 | 2 407 | 18.2 | 158 | 6.6 |
| ≥80 | 1 815 | 13.8 | 82 | 4.5 |
| Comorbidity | ||||
| 0 | 6 987 | 52.9 | 295 | 4.2 |
| 1 to 3 | 5 860 | 44.4 | 293 | 5.0 |
| ≥4 | 355 | 2.7 | 23 | 6.5 |
| Body mass index (kg/m2) | ||||
| Underweight (<18.5) | 192 | 1.5 | 4 | 2.1 |
| Ideal (18.5-24.9) | 3 099 | 23.5 | 93 | 3.0 |
| Overweight (25.0-29.9) | 2 520 | 19.1 | 148 | 5.9 |
| Obese (30.0-39.9) | 1 119 | 8.5 | 73 | 6.5 |
| Morbidly obese (≥40.0) | 440 | 3.3 | 38 | 8.6 |
| Missing | 5 832 | 44.2 | 255 | 4.4 |
| Surgery | ||||
| No | 3 093 | 23.4 | 104 | 3.4 |
| Yes | 10 109 | 76.6 | 507 | 5.0 |
| Chemotherapy | ||||
| No | 10 429 | 79.0 | 422 | 4.1 |
| Yes | 2 773 | 21.0 | 189 | 6.8 |
| Endocrine therapy | ||||
| No | 2 323 | 17.6 | 87 | 3.7 |
| Yes | 10 879 | 82.4 | 524 | 4.8 |
Demographic and tumor-related factors and risk of VTE
Increased age and BMI were significant predictors of VTE (Table 2). VTE risk increased with age, with women age 80 years or older at diagnosis having 5 times the risk compared with younger women (adjusted HR, 5.0; 95% CI, 3.0-8.2; AR, 14.9 per 1000 person-years). BMI had a similarly large influence on VTE risk, with the highest rate in women who were morbidly obese (BMI ≥40 kg/m2; HR, 3.0; 95% CI, 2.1-4.4; AR, 16.5; reference group, BMI 18.5-25.0 kg/m2). The rate of VTE was higher in women with metastatic cancer (18.2 per 1000 person-years) compared with those with local disease (6.8 per 1000 person-years). This was in part a result of the higher mean age among women with metastatic disease (66.3 years; standard deviation [SD], 15.1 years) compared with women who had local disease (61.3 years; SD, 12.8 years) and that more women with metastatic disease underwent chemotherapy (27.0%) than women with local disease (15.2%). When these two variables alone were adjusted for, the HR for metastatic disease decreased from 2.5 (95% CI, 1.6-4.0) in the univariable model to 1.5 (95% CI, 1.0-2.5). Similarly, a higher AR of VTE among women with a high Charlson score (≥4) could largely be accounted for by the higher mean age among women with a Charlson score ≥4 (72.0 years; SD, 13.0 years) than those with a Charlson score of 0 (60.6 years; SD, 14.5 years).
Rates of VTE in relation to potential risk factors
| . | No. of patients . | Events . | Person-years* . | AR . | Univariable Cox model . | Multivariable Cox model‡ . | |||
|---|---|---|---|---|---|---|---|---|---|
| Rate† . | 95% CI . | HR . | 95% CI . | HR . | 95% CI . | ||||
| Stage | |||||||||
| Local disease | 5 037 | 214 | 31.4 | 6.8 | 6.0-7.8 | Ref | Ref | ||
| Regional disease | 2 961 | 161 | 16.3 | 9.9 | 8.5-11.6 | 1.4 | 1.2-1.8 | 1.2 | 0.9-1.4 |
| Distant Metastases | 470 | 21 | 1.2 | 18.2 | 11.9-28.0 | 2.5 | 1.6-4.0 | 1.5 | 1.0-2.4 |
| Unknown | 4 734 | 215 | 23.8 | 9.0 | 7.9-10.3 | 1.3 | 1.1-1.6 | 1.2 | 0.9-1.4 |
| Grade | |||||||||
| Well differentiated | 1 681 | 71 | 11.1 | 6.4 | 5.1-8.1 | Ref | Ref | ||
| Moderately differentiated | 4 232 | 196 | 25.1 | 7.8 | 6.8-9.0 | 1.3 | 1.0-1.7 | 1.1 | 0.8-1.5 |
| Poorly differentiated | 3 024 | 166 | 16.1 | 10.3 | 8.9-12.0 | 1.2 | 0.9-1.6 | 1.1 | 0.8-1.4 |
| Unknown | 4 265 | 178 | 20.3 | 8.7 | 7.6-10.1 | 1.6 | 1.2-2.1 | 1.3 | 1.0-1.7 |
| Surgery | |||||||||
| No surgery | 3 093 | 104 | 11.5 | 9.0 | 7.4-10.9 | Ref | Ref | ||
| Before surgery | 13 | 1.5 | 8.7 | 5.1-15.0 | 0.9 | 0.5-1.6 | 0.7 | 0.4-1.2 | |
| During surgery hospitalization | 3 | 0.2 | 17.9 | 5.8-55.5 | 1.7 | 0.6-5.5 | 1.5 | 0.5-4.9 | |
| First month after discharge | 26 | 1.1 | 23.5 | 16.0-34.5 | 2.4 | 1.6-3.8 | 2.2 | 1.4-3.4 | |
| Second month after discharge | 24 | 1.0 | 24.3 | 16.3-36.2 | 2.4 | 1.5-3.8 | 1.4 | 0.9-2.2 | |
| Third month after discharge | 12 | 1.0 | 12.6 | 7.2-22.2 | 1.2 | 0.7-2.2 | 0.6 | 0.3-1.1 | |
| Subsequent time | 429 | 56.3 | 7.6 | 6.9-8.4 | 0.9 | 0.7-1.1 | 1.0 | 0.8-1.3 | |
| Chemotherapy | |||||||||
| No chemotherapy | 10 429 | 422 | 57.1 | 7.4 | 6.7-8.1 | Ref | Ref | ||
| Before chemotherapy | 31 | 2.7 | 11.7 | 8.2-16.6 | 1.5 | 1.0-2.1 | 1.6 | 1.1-2.4 | |
| During chemotherapy | 1.3 | 59.6 | 47.7-74.5 | 7.7 | 6.0-9.8 | 10.8 | 8.2-14.4 | ||
| First month after completion | 15 | 0.3 | 51.6 | 31.1-85.5 | 6.5 | 3.9-10.9 | 8.4 | 4.9-14.2 | |
| Second month after completion | 8 | 0.2 | 33.1 | 16.6-66.3 | 4.0 | 2.0-8.1 | 4.5 | 2.2-9.3 | |
| Third month after completion | 3 | 0.2 | 13.5 | 4.4-41.9 | 1.8 | 0.6-5.5 | 2.0 | 0.6-6.3 | |
| Subsequent time | 55 | 10.8 | 5.1 | 3.9-6.7 | 0.7 | 0.5-0.9 | 1.1 | 0.8-1.5 | |
| Endocrine therapy | |||||||||
| No endocrine therapy | 2 323 | 87 | 9.8 | 8.9 | 7.2-11.0 | Ref | Ref | ||
| Before endocrine therapy | 54 | 3.0 | 17.8 | 13.7-23.3 | 1.9 | 1.3-2.6 | 1.2 | 0.8-1.7 | |
| First 3 months of endocrine therapy | 69 | 2.5 | 27.7 | 21.9-35.0 | 2.9 | 2.1-4.0 | 2.4 | 1.7-3.4 | |
| Subsequent time | 401 | 57.3 | 7.0 | 6.3-7.7 | 0.8 | 0.6-1.0 | 0.9 | 0.7-1.1 | |
| Body mass index (kg/m2) | |||||||||
| Underweight (<18.5) | 192 | 4 | 1.1 | 3.8 | 1.4-10.1 | 0.7 | 0.3-2.0 | 0.7 | 0.3-1.9 |
| Ideal (18.5-24.9) | 3 099 | 93 | 18.3 | 5.1 | 4.2-6.2 | Ref | Ref | ||
| Overweight (25.0-29.9) | 2 520 | 148 | 14.7 | 10.1 | 8.6-11.8 | 2.0 | 1.5-2.6 | 1.8 | 1.4-2.4 |
| Obese (30.0-39.9) | 1 119 | 73 | 6.4 | 11.4 | 9.1-14.3 | 2.2 | 1.6-3.0 | 2.1 | 1.6-2.9 |
| Morbidly obese (≥40.0) | 440 | 38 | 2.3 | 16.5 | 12.0-22.7 | 3.2 | 2.2-4.7 | 3.0 | 2.1-4.4 |
| Missing | 5 832 | 255 | 29.9 | 8.5 | 7.6-9.7 | 1.6 | 1.3-2.0 | 1.5 | 1.2-1.9 |
| Age (years) | |||||||||
| <40 | 715 | 21 | 4.1 | 5.1 | 3.4-7.9 | Ref | Ref | ||
| 40-49 | 2 030 | 63 | 12.7 | 5.0 | 3.9-6.3 | 1.0 | 0.6-1.7 | 1.1 | 0.7-1.8 |
| 50-59 | 3 262 | 108 | 20.7 | 5.2 | 4.3-6.3 | 1.1 | 0.7-1.7 | 1.3 | 0.8-2.1 |
| 60-69 | 2 973 | 179 | 17.7 | 10.1 | 8.7-11.7 | 2.1 | 1.3-3.3 | 2.9 | 1.8-4.6 |
| 70-79 | 2 407 | 158 | 11.9 | 13.3 | 11.4-15.5 | 2.7 | 1.7-4.2 | 4.2 | 2.6-6.7 |
| ≥80 | 1 815 | 82 | 5.5 | 14.9 | 12.0-18.4 | 2.9 | 1.8-4.7 | 5.0 | 3.0-8.2 |
| Charlson score | |||||||||
| 0 | 6 987 | 295 | 37.5 | 7.8 | 7.1-8.8 | Ref | Ref | ||
| 1-3 | 5 860 | 293 | 33.6 | 8.7 | 7.8-9.8 | 1.1 | 1.0-1.4 | 1.0 | 0.8-1.1 |
| ≥4 | 355 | 23 | 1.5 | 15.4 | 10.2-23.1 | 2.1 | 1.4-3.3 | 1.3 | 0.9-2.1 |
| . | No. of patients . | Events . | Person-years* . | AR . | Univariable Cox model . | Multivariable Cox model‡ . | |||
|---|---|---|---|---|---|---|---|---|---|
| Rate† . | 95% CI . | HR . | 95% CI . | HR . | 95% CI . | ||||
| Stage | |||||||||
| Local disease | 5 037 | 214 | 31.4 | 6.8 | 6.0-7.8 | Ref | Ref | ||
| Regional disease | 2 961 | 161 | 16.3 | 9.9 | 8.5-11.6 | 1.4 | 1.2-1.8 | 1.2 | 0.9-1.4 |
| Distant Metastases | 470 | 21 | 1.2 | 18.2 | 11.9-28.0 | 2.5 | 1.6-4.0 | 1.5 | 1.0-2.4 |
| Unknown | 4 734 | 215 | 23.8 | 9.0 | 7.9-10.3 | 1.3 | 1.1-1.6 | 1.2 | 0.9-1.4 |
| Grade | |||||||||
| Well differentiated | 1 681 | 71 | 11.1 | 6.4 | 5.1-8.1 | Ref | Ref | ||
| Moderately differentiated | 4 232 | 196 | 25.1 | 7.8 | 6.8-9.0 | 1.3 | 1.0-1.7 | 1.1 | 0.8-1.5 |
| Poorly differentiated | 3 024 | 166 | 16.1 | 10.3 | 8.9-12.0 | 1.2 | 0.9-1.6 | 1.1 | 0.8-1.4 |
| Unknown | 4 265 | 178 | 20.3 | 8.7 | 7.6-10.1 | 1.6 | 1.2-2.1 | 1.3 | 1.0-1.7 |
| Surgery | |||||||||
| No surgery | 3 093 | 104 | 11.5 | 9.0 | 7.4-10.9 | Ref | Ref | ||
| Before surgery | 13 | 1.5 | 8.7 | 5.1-15.0 | 0.9 | 0.5-1.6 | 0.7 | 0.4-1.2 | |
| During surgery hospitalization | 3 | 0.2 | 17.9 | 5.8-55.5 | 1.7 | 0.6-5.5 | 1.5 | 0.5-4.9 | |
| First month after discharge | 26 | 1.1 | 23.5 | 16.0-34.5 | 2.4 | 1.6-3.8 | 2.2 | 1.4-3.4 | |
| Second month after discharge | 24 | 1.0 | 24.3 | 16.3-36.2 | 2.4 | 1.5-3.8 | 1.4 | 0.9-2.2 | |
| Third month after discharge | 12 | 1.0 | 12.6 | 7.2-22.2 | 1.2 | 0.7-2.2 | 0.6 | 0.3-1.1 | |
| Subsequent time | 429 | 56.3 | 7.6 | 6.9-8.4 | 0.9 | 0.7-1.1 | 1.0 | 0.8-1.3 | |
| Chemotherapy | |||||||||
| No chemotherapy | 10 429 | 422 | 57.1 | 7.4 | 6.7-8.1 | Ref | Ref | ||
| Before chemotherapy | 31 | 2.7 | 11.7 | 8.2-16.6 | 1.5 | 1.0-2.1 | 1.6 | 1.1-2.4 | |
| During chemotherapy | 1.3 | 59.6 | 47.7-74.5 | 7.7 | 6.0-9.8 | 10.8 | 8.2-14.4 | ||
| First month after completion | 15 | 0.3 | 51.6 | 31.1-85.5 | 6.5 | 3.9-10.9 | 8.4 | 4.9-14.2 | |
| Second month after completion | 8 | 0.2 | 33.1 | 16.6-66.3 | 4.0 | 2.0-8.1 | 4.5 | 2.2-9.3 | |
| Third month after completion | 3 | 0.2 | 13.5 | 4.4-41.9 | 1.8 | 0.6-5.5 | 2.0 | 0.6-6.3 | |
| Subsequent time | 55 | 10.8 | 5.1 | 3.9-6.7 | 0.7 | 0.5-0.9 | 1.1 | 0.8-1.5 | |
| Endocrine therapy | |||||||||
| No endocrine therapy | 2 323 | 87 | 9.8 | 8.9 | 7.2-11.0 | Ref | Ref | ||
| Before endocrine therapy | 54 | 3.0 | 17.8 | 13.7-23.3 | 1.9 | 1.3-2.6 | 1.2 | 0.8-1.7 | |
| First 3 months of endocrine therapy | 69 | 2.5 | 27.7 | 21.9-35.0 | 2.9 | 2.1-4.0 | 2.4 | 1.7-3.4 | |
| Subsequent time | 401 | 57.3 | 7.0 | 6.3-7.7 | 0.8 | 0.6-1.0 | 0.9 | 0.7-1.1 | |
| Body mass index (kg/m2) | |||||||||
| Underweight (<18.5) | 192 | 4 | 1.1 | 3.8 | 1.4-10.1 | 0.7 | 0.3-2.0 | 0.7 | 0.3-1.9 |
| Ideal (18.5-24.9) | 3 099 | 93 | 18.3 | 5.1 | 4.2-6.2 | Ref | Ref | ||
| Overweight (25.0-29.9) | 2 520 | 148 | 14.7 | 10.1 | 8.6-11.8 | 2.0 | 1.5-2.6 | 1.8 | 1.4-2.4 |
| Obese (30.0-39.9) | 1 119 | 73 | 6.4 | 11.4 | 9.1-14.3 | 2.2 | 1.6-3.0 | 2.1 | 1.6-2.9 |
| Morbidly obese (≥40.0) | 440 | 38 | 2.3 | 16.5 | 12.0-22.7 | 3.2 | 2.2-4.7 | 3.0 | 2.1-4.4 |
| Missing | 5 832 | 255 | 29.9 | 8.5 | 7.6-9.7 | 1.6 | 1.3-2.0 | 1.5 | 1.2-1.9 |
| Age (years) | |||||||||
| <40 | 715 | 21 | 4.1 | 5.1 | 3.4-7.9 | Ref | Ref | ||
| 40-49 | 2 030 | 63 | 12.7 | 5.0 | 3.9-6.3 | 1.0 | 0.6-1.7 | 1.1 | 0.7-1.8 |
| 50-59 | 3 262 | 108 | 20.7 | 5.2 | 4.3-6.3 | 1.1 | 0.7-1.7 | 1.3 | 0.8-2.1 |
| 60-69 | 2 973 | 179 | 17.7 | 10.1 | 8.7-11.7 | 2.1 | 1.3-3.3 | 2.9 | 1.8-4.6 |
| 70-79 | 2 407 | 158 | 11.9 | 13.3 | 11.4-15.5 | 2.7 | 1.7-4.2 | 4.2 | 2.6-6.7 |
| ≥80 | 1 815 | 82 | 5.5 | 14.9 | 12.0-18.4 | 2.9 | 1.8-4.7 | 5.0 | 3.0-8.2 |
| Charlson score | |||||||||
| 0 | 6 987 | 295 | 37.5 | 7.8 | 7.1-8.8 | Ref | Ref | ||
| 1-3 | 5 860 | 293 | 33.6 | 8.7 | 7.8-9.8 | 1.1 | 1.0-1.4 | 1.0 | 0.8-1.1 |
| ≥4 | 355 | 23 | 1.5 | 15.4 | 10.2-23.1 | 2.1 | 1.4-3.3 | 1.3 | 0.9-2.1 |
Subsequent time refers to the time and rate after the procedure until the completion of follow-up.
Ref, reference.
Divided by 1000.
Per 1000 person-years.
HRs adjusted for all other variables in the table. All variables were fitted by using the categorization displayed in the table (ie, age group as a 6-level nonordered categorical variable).
Surgery and risk of VTE
Surgery in this cohort took the form of either mastectomy (with 9.1% having immediate reconstruction) or a breast-conserving procedure. When other factors were accounted for, a significantly increased risk compared with those not undergoing surgery existed only in the first month after discharge from the surgical admission (HR, 2.2; 95% CI, 1.4-3.4; AR, 23.5 per 1000 person-years) (Table 2). Rates of VTE after surgery did not vary by stage (monthly rates ranged from 17 to 28 per 1000 person-years; Table 3; test for interaction between surgery and stage, P = .15).
Rates of VTE by stage stratified by treatment
| . | Events . | Person-years* . | Rate† . | 95% CI . | Univariable . | Multivariable‡ . | ||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | HR . | 95% CI . | |||||
| No active therapy§ | ||||||||
| Local disease | 160 | 29.99 | 5.3 | 4.6-6.2 | Ref | Ref | ||
| Regional disease | 113 | 14.94 | 7.6 | 6.3-9.1 | 1.4 | 1.1-1.8 | 1.4 | 1.1-1.8 |
| Distant metastases | 18 | 1.03 | 17.5 | 11.0-27.7 | 3.2 | 2.0-5.2 | 2.5 | 1.6-4.2 |
| Unknown | 170 | 22.67 | 7.5 | 6.5-8.7 | 1.4 | 1.1-1.8 | 1.2 | 1.0-1.6 |
| Active chemotherapy|| | ||||||||
| Local disease | 27 | 0.43 | 63.0 | 43.2-91.9 | Ref | Ref | ||
| Regional disease | 38 | 0.74 | 51.3 | 37.3-70.5 | 0.8 | 0.5-1.3 | 0.8 | 0.5-1.2 |
| Distant metastases | —¶ | —¶ | 26.2 | 6.6-104.8 | 0.4 | 0.1-1.7 | 0.3 | 0.1-1.4 |
| Unknown | 30 | 0.46 | 65.7 | 45.9-93.9 | 1.1 | 0.6-1.8 | 1.0 | 0.6-1.7 |
| Active surgery# | ||||||||
| Local disease | 26 | 0.92 | 28.1 | 19.2-41.3 | Ref | Ref | ||
| Regional disease | 9 | 0.52 | 17.2 | 8.9-33.0 | 0.6 | 0.3-1.3 | 0.6 | 0.3-1.4 |
| Distant metastases | —¶ | —¶ | 24.2 | 3.4-171.5 | 0.9 | 0.1-6.4 | 0.8 | 0.1-5.7 |
| Unknown | 14 | 0.65 | 21.5 | 12.7-36.3 | 0.8 | 0.4-1.5 | 0.7 | 0.4-1.4 |
| . | Events . | Person-years* . | Rate† . | 95% CI . | Univariable . | Multivariable‡ . | ||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | HR . | 95% CI . | |||||
| No active therapy§ | ||||||||
| Local disease | 160 | 29.99 | 5.3 | 4.6-6.2 | Ref | Ref | ||
| Regional disease | 113 | 14.94 | 7.6 | 6.3-9.1 | 1.4 | 1.1-1.8 | 1.4 | 1.1-1.8 |
| Distant metastases | 18 | 1.03 | 17.5 | 11.0-27.7 | 3.2 | 2.0-5.2 | 2.5 | 1.6-4.2 |
| Unknown | 170 | 22.67 | 7.5 | 6.5-8.7 | 1.4 | 1.1-1.8 | 1.2 | 1.0-1.6 |
| Active chemotherapy|| | ||||||||
| Local disease | 27 | 0.43 | 63.0 | 43.2-91.9 | Ref | Ref | ||
| Regional disease | 38 | 0.74 | 51.3 | 37.3-70.5 | 0.8 | 0.5-1.3 | 0.8 | 0.5-1.2 |
| Distant metastases | —¶ | —¶ | 26.2 | 6.6-104.8 | 0.4 | 0.1-1.7 | 0.3 | 0.1-1.4 |
| Unknown | 30 | 0.46 | 65.7 | 45.9-93.9 | 1.1 | 0.6-1.8 | 1.0 | 0.6-1.7 |
| Active surgery# | ||||||||
| Local disease | 26 | 0.92 | 28.1 | 19.2-41.3 | Ref | Ref | ||
| Regional disease | 9 | 0.52 | 17.2 | 8.9-33.0 | 0.6 | 0.3-1.3 | 0.6 | 0.3-1.4 |
| Distant metastases | —¶ | —¶ | 24.2 | 3.4-171.5 | 0.9 | 0.1-6.4 | 0.8 | 0.1-5.7 |
| Unknown | 14 | 0.65 | 21.5 | 12.7-36.3 | 0.8 | 0.4-1.5 | 0.7 | 0.4-1.4 |
Divided by 1000.
Per 1000 person-years.
Adjusted for grade, endocrine therapy, BMI, comorbidity (Charlson score), and age. These variables were fitted using the same categorization as in multivariable models presented in Table 2.
Cohort time during which participants are not at risk as a result of surgery or chemotherapy.
During chemotherapy and for the 2 months after completion of therapy. Excludes person-time during which participants are at risk after surgery.
Number of outcome events and person-time are censored for cell frequencies <5, in line with CPRD policy.
During surgery and for the first 2 months after discharge from the surgical hospital. Excludes person-time during which participants are at risk during or after chemotherapy.
Chemotherapy and risk of VTE
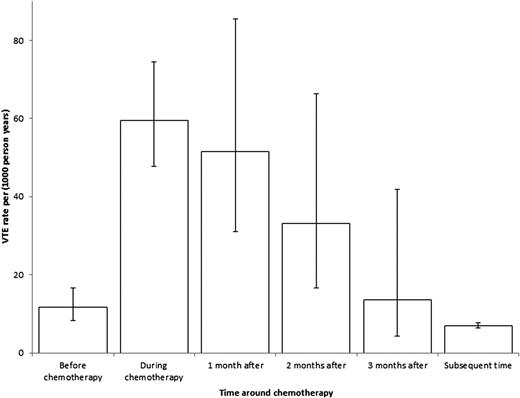
Women who underwent chemotherapy had very high ARs of VTE both during chemotherapy and in the month after cessation of therapy (both >50 per 1000 person-years) (Table 2). The adjusted HRs compared with no chemotherapy were 10.8 (95% CI, 8.2 to 14.4) during chemotherapy and 8.4 (95% CI, 4.9-14.2) in the month afterward. The risk of VTE remained high in the second month after completion of therapy, but by 3 months, the risk had reverted to that before treatment (Figure 1). The adjusted HR during chemotherapy compared with the time before chemotherapy in the same patients was 6.6 (95% CI, 4.3-10.1). The effect of stage on risk of VTE was more pronounced during follow-up time outside chemotherapy (HR, 2.5 for metastatic disease; 95% CI, 1.6 to 4.2), with a significant interaction between stage and chemotherapy treatment (P = .004) (Table 3).
Independent effects of stage, surgery, and chemotherapy
When looking at chemotherapy and surgery together, the AR of VTE was particularly high when surgery took place in the 2-month chemotherapy recovery period; however, this was based on a small number of events and thus the CI was wide (AR, 92.1; 95% CI, 38.3-221.2) (Table 4).
Rates of VTE by surgery and chemotherapy
| . | Events . | Person-years . | AR* . | 95% CI . |
|---|---|---|---|---|
| Baseline† | 450 | 67 600 | 6.7 | 6.1-7.3 |
| Surgery only‡ | ||||
| During surgery hospitalization | —§ | —§ | 12.2 | 3.1-48.9 |
| 1st month after discharge | 24 | 1 075 | 22.3 | 15.0-33.3 |
| 2nd month after discharge | 24 | 893 | 26.9 | 18.0-40.1 |
| 3rd month after discharge | 8 | 818 | 9.8 | 4.9-19.6 |
| Chemotherapy only|| | ||||
| During chemotherapy | 75 | 1 080 | 69.4 | 55.4-87.1 |
| 1 mo after chemotherapy | 13 | 271 | 48.0 | 27.9-82.6 |
| 2 mo after chemotherapy | 5 | 224 | 22.3 | 9.3-53.7 |
| 3 mo after chemotherapy | —§ | —§ | 14.6 | 4.7-45.3 |
| Surgery and chemotherapy | ||||
| Chemotherapy during surgery recovery | —§ | —§ | 9.4 | 2.4-37.6 |
| Surgery during chemotherapy recovery¶ | 5 | 54 | 92.1 | 38.3-221.2 |
| . | Events . | Person-years . | AR* . | 95% CI . |
|---|---|---|---|---|
| Baseline† | 450 | 67 600 | 6.7 | 6.1-7.3 |
| Surgery only‡ | ||||
| During surgery hospitalization | —§ | —§ | 12.2 | 3.1-48.9 |
| 1st month after discharge | 24 | 1 075 | 22.3 | 15.0-33.3 |
| 2nd month after discharge | 24 | 893 | 26.9 | 18.0-40.1 |
| 3rd month after discharge | 8 | 818 | 9.8 | 4.9-19.6 |
| Chemotherapy only|| | ||||
| During chemotherapy | 75 | 1 080 | 69.4 | 55.4-87.1 |
| 1 mo after chemotherapy | 13 | 271 | 48.0 | 27.9-82.6 |
| 2 mo after chemotherapy | 5 | 224 | 22.3 | 9.3-53.7 |
| 3 mo after chemotherapy | —§ | —§ | 14.6 | 4.7-45.3 |
| Surgery and chemotherapy | ||||
| Chemotherapy during surgery recovery | —§ | —§ | 9.4 | 2.4-37.6 |
| Surgery during chemotherapy recovery¶ | 5 | 54 | 92.1 | 38.3-221.2 |
Per 1000 person-years.
Includes all cohort time except for the time during and 3 months after chemotherapy and during hospitalization for surgery and 3 months after discharge. This includes the entire study time for women who did not undergo either chemotherapy or surgery.
Excludes time during and 3 months after chemotherapy.
Number of outcome events and person-time are censored for cell frequencies <5, in line with CPRD policy.
Excludes time during hospitalization for surgery and 3 months after discharge.
Includes time during hospitalization for surgery and the 3 months after discharge.
Endocrine therapy and risk of VTE
For women who received endocrine therapy, the risk of VTE in the 3 months after beginning therapy was more than double the risk in those who did not receive endocrine therapy (HR, 2.4; 95% CI, 1.7-3.4; AR, 27.7) (Table 2). No increased risk was observed beyond 3 months of therapy (HR, 0.9; 95% CI, 0.7-1.1; AR, 7.0).
When ARs were explored on the basis of type of endocrine therapy, there were important differences between tamoxifen and AIs (Table 5). VTE risk increased more than fivefold in the 3 months after initiating therapy among women who received tamoxifen only (HR, 5.5; 95% CI, 2.3-12.7; AR, 24.1; reference category, risk before beginning tamoxifen). After 3 months, a nonsignificant twofold increase in risk remained (HR, 1.9; 95% CI, 0.9-4.3; AR, 5.2). Specific adjustment for chemotherapy (which occurred frequently in the cohort time prior to tamoxifen therapy) accounted for most of the difference between the univariable and multivariable results (HR, 1.1 vs 1.9). In contrast to those receiving tamoxifen, among those receiving an AI only, there was no increase in risk in the 3 months after starting therapy (HR, 0.8; 95% CI, 0.5-1.4; AR, 28.3; reference category, risk before starting AIs). Furthermore, we found that among women who switched to an AI after initially receiving tamoxifen, the AR after beginning the AI was similar to that in the period before beginning the AI (subsequent time for tamoxifen; Table 5).
Rates of VTE by type of endocrine therapy
| . | No. of patients . | Events . | Person-years . | AR . | Univariable Cox model . | Multivariable Cox model† . | |||
|---|---|---|---|---|---|---|---|---|---|
| Rate* . | 95% CI . | HR . | 95% CI . | HR . | 95% CI . | ||||
| Tamoxifen only | 5415 | ||||||||
| Before therapy | 7 | 1 198 | 5.8 | 2.8-12.3 | Ref | Ref | |||
| First 3 months‡ | 30 | 1 246 | 24.1 | 16.8-34.4 | 4.1 | 1.8-9.4 | 5.5 | 2.3-12.7 | |
| Subsequent time | 159 | 30 453 | 5.2 | 4.5-6.1 | 1.1 | 0.5-2.9 | 1.9 | 0.9-4.3 | |
| Aromatase inhibitors only | 1410 | ||||||||
| Before therapy | 28 | 627 | 44.6 | 30.8-64.7 | Ref | Ref | |||
| First 3 months† | 9 | 318 | 28.3 | 14.7-54.3 | 0.6 | 0.3-1.3 | 0.8 | 0.5-1.4 | |
| Subsequent time | 46 | 4 697 | 9.8 | 7.3-13.1 | 0.2 | 0.1-0.4 | 0.3 | 0.2-0.5 | |
| Tamoxifen followed by AIs§ | 3821 | ||||||||
| Before tamoxifen | 18 | 1 121 | 16.1 | 10.1-25.5 | Ref | Ref | |||
| First 3 months of tamoxifen|| | 29 | 859 | 33.8 | 23.5-48.6 | 2.1 | 1.2-3.8 | 2.3 | 1.2-4.4 | |
| Subsequent time for tamoxifen|| | 97 | 8 812 | 11.0 | 9.0-13.4 | 0.7 | 0.4-1.1 | 1.1 | 0.6-2.0 | |
| First 3 months of AIs | 10 | 852 | 11.7 | 6.3-21.8 | 0.7 | 0.3-1.6 | 1.0 | 0.4-2.4 | |
| Subsequent time for AIs¶ | 78 | 11 509 | 6.8 | 5.4-8.5 | 0.4 | 0.2-0.7 | 0.6 | 0.3-1.2 | |
| . | No. of patients . | Events . | Person-years . | AR . | Univariable Cox model . | Multivariable Cox model† . | |||
|---|---|---|---|---|---|---|---|---|---|
| Rate* . | 95% CI . | HR . | 95% CI . | HR . | 95% CI . | ||||
| Tamoxifen only | 5415 | ||||||||
| Before therapy | 7 | 1 198 | 5.8 | 2.8-12.3 | Ref | Ref | |||
| First 3 months‡ | 30 | 1 246 | 24.1 | 16.8-34.4 | 4.1 | 1.8-9.4 | 5.5 | 2.3-12.7 | |
| Subsequent time | 159 | 30 453 | 5.2 | 4.5-6.1 | 1.1 | 0.5-2.9 | 1.9 | 0.9-4.3 | |
| Aromatase inhibitors only | 1410 | ||||||||
| Before therapy | 28 | 627 | 44.6 | 30.8-64.7 | Ref | Ref | |||
| First 3 months† | 9 | 318 | 28.3 | 14.7-54.3 | 0.6 | 0.3-1.3 | 0.8 | 0.5-1.4 | |
| Subsequent time | 46 | 4 697 | 9.8 | 7.3-13.1 | 0.2 | 0.1-0.4 | 0.3 | 0.2-0.5 | |
| Tamoxifen followed by AIs§ | 3821 | ||||||||
| Before tamoxifen | 18 | 1 121 | 16.1 | 10.1-25.5 | Ref | Ref | |||
| First 3 months of tamoxifen|| | 29 | 859 | 33.8 | 23.5-48.6 | 2.1 | 1.2-3.8 | 2.3 | 1.2-4.4 | |
| Subsequent time for tamoxifen|| | 97 | 8 812 | 11.0 | 9.0-13.4 | 0.7 | 0.4-1.1 | 1.1 | 0.6-2.0 | |
| First 3 months of AIs | 10 | 852 | 11.7 | 6.3-21.8 | 0.7 | 0.3-1.6 | 1.0 | 0.4-2.4 | |
| Subsequent time for AIs¶ | 78 | 11 509 | 6.8 | 5.4-8.5 | 0.4 | 0.2-0.7 | 0.6 | 0.3-1.2 | |
Per 1000 person-years.
Adjusted for stage, grade, surgery, chemotherapy, BMI, comorbidity (Charlson score), and age. These variables were fitted by using the same categorization as in multivariable models presented in Table 2.
Three months from day of the first tamoxifen or AI prescription after cancer diagnosis.
An equivalent analysis was not presented for the 233 women (12 VTE events) who switched from AIs to tamoxifen because this number was too small for a meaningful analysis.
Exposure period ceases on the day on which patients switch to AI (if applicable).
Cohort time starting 3 months after initiation of AI therapy until the completion of follow-up.
Discussion
It is known that women with breast cancer have a 3.5-fold increased risk of VTE compared with women of a similar age without cancer.1,2 A risk of this magnitude would not justify continuous VTE prophylaxis, and thus it becomes important to identify subgroups of patients and time during which the risk is highest. By using data that are routinely available from primary care, secondary care, and United Kingdom cancer registries, we report high risks of VTE among women undergoing chemotherapy and in the first 3 months of treatment with tamoxifen. During chemotherapy, the risk of VTE was increased more than 10-fold, and this risk remained high in the 2 months after completing treatment. Among women who received tamoxifen, the risk of VTE was 5 times higher than it was in the period before starting therapy. We also observed a doubling of rates of VTE in women with breast cancer over the 10-year study period. This is consistent with reports of an increase in risk of cancer-associated VTE over time in this and in other populations, a trend which may be the result of either more aggressive cancer treatments or ascertainment resulting from greater knowledge of the link between cancer and thrombosis.1,20-22
This report contained both absolute risks (unadjusted) and adjusted HRs. Absolute risks and associated unadjusted HRs may be more useful in terms of clinical decision making if they are interpreted on the basis of a single factor. However, we observed that unadjusted and adjusted HRs were always similar except in 2 specific instances: with chemotherapy and stage and with age and comorbidity. In a stratified analysis involving chemotherapy and stage, we reported a much higher risk of VTE with metastatic disease compared with local disease within time periods not influenced by chemotherapy (unadjusted HR, 3.2; adjusted HR, 2.5). However, during chemotherapy and immediately afterward, rates of VTE were high regardless of stage, although these estimates were based on small event numbers.
Our observed rate of 8.4 per 1000 person-years was similar to that reported by Chew et al7 in the largest study carried out to date on VTE risk in breast cancer. In our study, we were able to examine several risk factors absent from the California study, including detailed and accurate recording of chemotherapy treatment and use of endocrine therapy as well as BMI. Furthermore, our study contained a longer average follow-up (5.5 years) than the California and other comparable studies,2,8,9 and we were able to assess the effects of surgery and chemotherapy in a time-dependent manner in a way different from that used in previous research.
Chemotherapy is known to be an important risk factor for VTE in patients with cancer. Previously, in relatively small cohorts of women receiving chemotherapy for breast cancer, risks of VTE have ranged from 5.5% to 8.0% among women with locoregional disease5,23,24 and from 4.4% to 17.7% in women with metastatic disease.23,25,26 By using data on 2773 women undergoing chemotherapy for breast cancer, we reported a risk of VTE that was the equivalent of 5% to 6% per year during chemotherapy and in the month after therapy (3.7% of women undergoing chemotherapy developed a VTE either during treatment or in the 3 months after completion of treatment). The risk during therapy was 6.6 times higher than in the same women before therapy when adjusted for other treatment factors. A validated risk prediction tool has been developed to determine which patients undergoing chemotherapy are at highest risk and could therefore benefit from prophylaxis.27 However, neither that study nor a more recent extension28 of the score was able to demonstrate the periods of highest risk for patients, and because both required informed consent, they were likely to exclude those with the poorest performance status.
We found that the risk of VTE was approximately twofold higher following discharge after surgery; however, the risk was increased only for the month after surgery once other factors were accounted for, and the magnitude of the increase was much less than that after chemotherapy. This smaller effect may in part reflect the minimal invasiveness of breast cancer surgery which does not penetrate body cavities, particularly in an era when breast reconstruction was less frequently performed. Although 70% to 80% of breast surgeons from the United Kingdom in this era prescribed anticoagulant thromboprophylaxis to breast cancer patients undergoing surgery, our increased risk was observed during the time after discharge in which thromboprophylaxis is not usually administered.29 Despite limited numbers, we demonstrate a particularly increased risk of VTE associated with surgery after chemotherapy. Neoadjuvant chemotherapy is increasingly being used, which has important implications for surgical thromboprophylaxis in the neoadjuvant setting.
Tamoxifen therapy is a widely recognized risk factor for VTE. The magnitude of increase in risk of VTE we observed after beginning tamoxifen therapy was comparable to that in studies of women with early-stage breast cancer between 1989 and 1999, which report an excess risk of VTE events in women receiving tamoxifen between 1.5-fold and sevenfold.30 Our results are also concordant with those from a large cohort of women from Denmark with early-stage breast cancer in which women treated with tamoxifen had a 3.5-fold higher risk of VTE than women receiving other treatments.10 Previous research has found the influence of tamoxifen on risk of VTE to attenuate 1 to 2 years after beginning therapy,10,31 possibly suggesting an adaptation of the hemostatic system to the procoagulant effects of tamoxifen. This is the first study to demonstrate that the prothrombotic effects of tamoxifen are noticeably reduced after only 3 months of treatment. More recently, AIs have become more commonly used; however, the impact of AI use on the risk of VTE is less clearly established.32 In observational research such as in this study, a direct comparison of VTE risks between tamoxifen and AIs is complicated by the fact that prescriptions for AIs were until quite recently limited to higher-risk patients because of the high cost of on-patent drugs, as evidenced by the very high baseline rate of VTE among this group. In addition, patients receiving AIs are likely to be an older group, because use of AIs, unlike use of tamoxifen, is limited to postmenopausal women.
A limitation of our study is that although we had a large sample size overall, examining risks on the basis of more intricate combinations of risk factors was hampered by small numbers of events. For instance, although we found that the risk of VTE was high in women undergoing surgery shortly after chemotherapy, only 5 VTE events occurred during this period and hence the CI around the absolute risk was wide. Our results also need to be interpreted in light of the fact that data on cancer stage could not be determined for about one third of our sample. This reflects our reliance on routinely collected data in which this level of recording is standard. However, among those who did have data on stage, 5-year survival rates among women with localized (88%) and metastatic (27%) disease in our study were consistent with rates in published data from the US population.33 As we have previously acknowledged, our algorithm for defining VTE was not validated specifically in cancer patients and would not capture anticoagulant prescriptions emanating from secondary care.1 However, the usual requirement is for cancer patients to receive continuous anticoagulant therapy for a minimum of 3 months after the primary VTE, and such prescriptions would be captured in primary care. Furthermore, previous studies that used administrative data have relied solely on physician coding of VTE without requiring evidence of anticoagulation.7,8,10,34 Previously, our algorithm was shown to have a positive predictive value of 84% when using VTE codes from primary care only.19 Although this value is higher than that in studies that relied solely on administrative codes for defining VTE,35-37 it could still indicate that some of our VTE cases are liable to be false positives which, if greater than the number of VTE cases not captured by our algorithm, would result in a slight overestimation in absolute risks of VTE in each exposure category. Another limitation of our work is the lack of data on thromboprophylaxis occurring during inpatient episodes, whether for cancer treatments (eg, surgery) or complications of the malignancy. However, thromboprophylaxis specifically for systemic cancer treatment was rare based on a questionnaire survey conducted during this study period.38 In addition, aspirin for ambulatory thromboprophylaxis is likely to have been obtained predominantly over the counter for this study cohort (median age, 62 years), so this would also not be reliably captured via our primary care data. Finally, we relied on adjustment for Charlson score to account for any confounding that resulted from underlying health status. Although one could argue that this would be less exhaustive than adjusting for specific health indices that influence VTE risk, we found that adjusting for Charlson score had very minimal impact on the effect sizes observed for other variables of interest. Consequently, we believe it is very unlikely that adjustment for additional health-related variables would explain much of the large increase in risk we observed after chemotherapy and initiation of tamoxifen unless the Charlson score was a particularly poor indicator of morbidity which has been proved not to be the case.39
Breast cancer is the most common cancer worldwide, with women in the United Kingdom specifically having a 1-in-8 lifetime risk of developing breast cancer.40 The financial and human impact of breast cancer–related VTE therefore means that it is vital to identify women who are most at risk and would potentially benefit from prophylactic intervention. Our data enabled us to address this by demonstrating that although chemotherapy is known to increase the risk of VTE in breast and other cancers, this risk remained high for 2 months after completing the final course of therapy, and that this risk may be increased further when chemotherapy is started soon after surgery. Such detail can complement the recent American College of Chest Physicians and National Comprehensive Cancer Network guidelines recommending prophylaxis for higher-risk outpatient people with cancer.12,13 Speculation on the absolute VTE risk above which prophylaxis is advised needs to take into account the harms (bleeding in particular) as well as benefits of prolonged anticoagulation, which is beyond the scope of this study. Further observational research on this topic would benefit from the inclusion of information on laboratory data (eg, hemoglobin and platelet counts) as well as more novel biomarkers which are not universally available in studies that use electronic data recorded for administrative purposes. One benefit of our study, however, is that risks are presented on the basis of easily ascertainable yet time-dependent risk factors, which we believe could form the basis for selecting appropriate patients for future randomized trials. Future research should focus on the development of prognostic models to identify specific women with breast cancer for whom the benefits of prophylactic intervention would outweigh the harms.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Population and Research Committee project grant C17683/A12079 from Cancer Research UK. J.W. is funded by a University of Nottingham/Nottingham University Hospitals National Health Service Trust Senior Clinical Research Fellowship. C.C.K. is funded by a National Institute for Health Research Clinician Scientist Award.
Authorship
Contribution: J.W., T.R.C., and M.J.G. were responsible for conceiving and designing the study; A.J.W. was primarily responsible for data management with input from C.C.; A.J.W. performed the data analysis under the supervision of M.J.G.; C.C.K. provided advice relating to clinical aspects during all stages of the project (especially during planning analyses and interpreting results); A.J.W. and M.J.G. wrote the first draft of the manuscript; all authors contributed to subsequent versions of the manuscript; and all authors have seen and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew J. Grainge, Division of Epidemiology and Public Health, School of Medicine, University of Nottingham, Clinical Sciences Building 2, City Hospital, Nottingham NG5 1PB, United Kingdom; e-mail: matthew.grainge@nottingham.ac.uk.