Abstract
Effective medical management for sickle cell disease (SCD) remains elusive. As a prevalent and severe monogenic disorder, SCD has been long considered a logical candidate for gene therapy. Significant progress has been made in moving toward this goal. These efforts have provided substantial insight into the natural regulation of the globin genes and illuminated challenges for genetic manipulation of the hematopoietic system. The initial γ-retroviral vectors, next-generation lentiviral vectors, and novel genome engineering and gene regulation approaches each share the goal of preventing erythrocyte sickling. After years of preclinical studies, several clinical trials for SCD gene therapies are now open. This review focuses on progress made toward achieving gene therapy, the current state of the field, consideration of factors that may determine clinical success, and prospects for future development.
Introduction
The single base substitution A-T (β6Glu→Val) in the first exon of the β-globin gene is the defining mutation of the sickle allele. Individuals homozygous for the mutation have the classical sickle cell disease (SCD) genotype. All pathophysiological consequences ensue from this monogenic defect. The amino acid substitution in βS-globin allows formation of defective hemoglobin tetramers that polymerize upon deoxygenation.1 Hemoglobin polymerization causes affected red blood cells (RBCs) to lose normal deformability and adopt the archetypal sickle shape. Rigid sickle RBCs prematurely breakdown, damage endothelia, and occlude vasculature, leading to a cascade of hemolysis, ischemia, inflammation, and endothelial injury.2 Patients are afflicted with intensely painful episodes, susceptibility to infection, end-organ injury, and early mortality among other sequelae.3,4 Despite its status as a “monogenic” disease, SCD is surprisingly variable in its clinical severity.
At present, the only curative treatment of SCD is allogeneic hematopoietic stem cell (HSC) transplantation. 6-year disease-free survival of >90% has been reported for transplants from HLA-matched sibling donors.5 However, in the United States, <14% of patients have a matched sibling donor.6 Transplants with matched unrelated donors are limited by donor availability and immunologic barriers, such as graft rejection and graft-versus-host disease.3 Attempts to extend allogeneic transplant for SCD to alternative donor sources is an area of ongoing effort.7 The SCD community has been cautious to embrace allogeneic HSC transplant in part given its short-term morbidity and mortality risks, though nonmyeloablative preparative regimens may help mitigate these risks.8-11 Given that the current clinical approach to SCD is largely reliant upon supportive care and hydroxyurea,12 the development of definitive therapies based on genetic manipulation of autologous HSCs would constitute a major advance.
Gene therapy has long been proposed as a potential cure for SCD.13 The permanent delivery of a corrective or antisickling gene cassette into long-term, repopulating HSCs could allow for the production of corrected RBCs for the life of the patient. More recent advances in manipulating the human genome suggest nonviral forms of genome editing could also be therapeutically relevant. This review will focus on the initial development of viral vectors for SCD gene therapy via gene addition, discuss current vectors in detail including those in clinical trials, highlight novel approaches to SCD genetic manipulation, and discuss key parameters for achieving clinical success. Although outside the scope of this article, it is notable that many of the approaches discussed below are also relevant to β-thalassemia, a disorder of inadequate β-globin production.
Gene addition strategies
Over the last 3 decades, several groups have worked toward achieving efficient and safe gene transfer to HSCs for SCD as well as other genetic disorders.14 In order for gene therapy for SCD to become a reality, 2 main objectives must be achieved: (1) safe and efficient gene transfer or correction of long-term repopulating HSCs and (2) high-level, appropriately regulated, stable gene expression. With current progress at the bench and in the clinic, these goals now appear within reach. The long path to the clinic for SCD gene therapies has been paved by landmark discoveries that have provided important insights into the developmental regulation of the β-globin gene cluster.
β-globin–expressing vectors
Initial attempts to develop integrating γ-retroviral vectors for β-globin gene transfer revealed challenges to attaining efficient, high-level erythroid-specific expression following HSC transduction. Long terminal repeat (LTR)-driven transgene expression of β-globin resulted in little or no β-globin following hematopoietic reconstitution in mice.15-18 The principal finding that allowed for high-level erythroid-specific expression in vivo was the discovery and characterization of the β-globin locus control region (LCR). This essential regulatory element 40 to 60 kb upstream of the β-globin gene, which is demarcated by 5 DNase I hypersensitive (HS) sites, contains potent erythroid-specific enhancers that function in concert with elements flanking the downstream globin genes.19-21 Careful mapping of the positions and activities of these HS sites established the need for powerful erythroid-specific enhancer elements for high-level erythroid expression.19,22-27
A second challenge encountered with retroviral vectors was the recognition that they are subject to expression variegation and silencing.28,29 Furthermore, their powerful regulatory elements may alter expression of endogenous genes, particularly those adjacent to their insertion site.30 Insulators that shield internal sequences from repressive effects of neighboring chromatin (barrier activity) or decrease trans-activation by enhancers (enhancer-blocking activity), such as the chicken hypersensitive site-4 (cHS4), ankyrin, and FB (second DNaseI hypersensitive site from 5′ of cHS4 [FII] and blocking element alpha/delta 1 [BEAD]) insulators, may promote the safety and efficacy of integrating vectors.31-38 Balancing the benefits of expression control with the negative impact of large insulators on vector titer has been an ongoing issue. Recently, small enhancer-blocking insulators have been described that do not diminish viral titer in reporter constructs.39
As evidence accumulated that lentiviral vectors offer important advantages over γ-retroviral vectors (including the ability to transduce nondividing HSCs, a larger capacity for DNA, stable transmission of complicated cargo, and a safer integration profile), the gene therapy field has largely transitioned to the use of these vectors.14,40-45 The tendency for γ-retroviral vectors to integrate near the transcriptional start sites of genes exacerbates their potential to alter gene expression. In contrast, lentiviruses tend to insert more randomly with a bias toward integration in gene bodies of expressed genes.43,46 In addition, vectors with a self-inactivating (SIN) design have become standard. SIN vectors have a deletion in the U3 region of the 3′ LTR, which is copied into the 5′ LTR upon reverse transcription. This modification minimizes transactivation of nearby genes, thereby limiting potential for insertional oncogenesis47 (Figure 1). Various lentiviral vectors carrying human β-globin have been used to correct disease models in both β-thalassemia and SCD.48-51
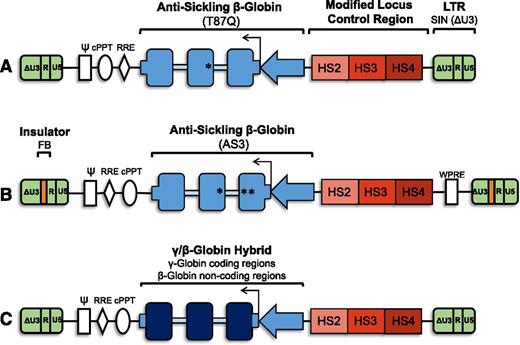
Vector schematic: general organization of proviral form of lentiviral vectors for gene therapy for SCD. (A) Antisickling β-globin vector containing the T87Q mutation. The SIN lentiviral vector is noninsulated and contains a modified LCR with HS2, HS3, and HS4. (B) Antisickling β-globin vector containing the G16D, E22A, and T87Q mutations (AS3). The SIN lentiviral vector has an FB element for enhancer-blocking activity and contains a modified LCR with HS2, HS3, and HS4. (C) A γ/β-globin hybrid vector containing the coding regions of γ-globin and the noncoding regions of β-globin. γ-globin (dark blue boxes), β-globin, or antisickling β-globin gene cassettes (blue boxes) with β-globin 5′ and 3′ untranslated regions (short blue boxes) under the control of a β-globin promoter (blue arrow) and modified β-globin LCR (red). Gene cassette is in reverse with respect to viral transcription to avoid aberrant splicing during packaging due to presence of globin intronic sequences (light blue boxes). All vectors are SIN (ΔU3) lentiviral vectors (green). Vectors are intended as basic schematics of gene therapy clinical vectors for SCD and are neither drawn to scale nor are all details included. ψ, packaging signal; cPPT, central polypurine tract; HS, DNase I hypersensitive site; LTR, long terminal repeats (U3, R, and U5); RRE, rev-responsive element; WPRE, woodchuck hepatitis virus posttranscriptional regulator element.
Vector schematic: general organization of proviral form of lentiviral vectors for gene therapy for SCD. (A) Antisickling β-globin vector containing the T87Q mutation. The SIN lentiviral vector is noninsulated and contains a modified LCR with HS2, HS3, and HS4. (B) Antisickling β-globin vector containing the G16D, E22A, and T87Q mutations (AS3). The SIN lentiviral vector has an FB element for enhancer-blocking activity and contains a modified LCR with HS2, HS3, and HS4. (C) A γ/β-globin hybrid vector containing the coding regions of γ-globin and the noncoding regions of β-globin. γ-globin (dark blue boxes), β-globin, or antisickling β-globin gene cassettes (blue boxes) with β-globin 5′ and 3′ untranslated regions (short blue boxes) under the control of a β-globin promoter (blue arrow) and modified β-globin LCR (red). Gene cassette is in reverse with respect to viral transcription to avoid aberrant splicing during packaging due to presence of globin intronic sequences (light blue boxes). All vectors are SIN (ΔU3) lentiviral vectors (green). Vectors are intended as basic schematics of gene therapy clinical vectors for SCD and are neither drawn to scale nor are all details included. ψ, packaging signal; cPPT, central polypurine tract; HS, DNase I hypersensitive site; LTR, long terminal repeats (U3, R, and U5); RRE, rev-responsive element; WPRE, woodchuck hepatitis virus posttranscriptional regulator element.
γ-Globin and other antisickling vectors
In addition to vectors containing β-globin expression cassettes, vectors containing alternative globin genes, such as γ-globin or hybrid β/γ-globin, have been developed. The rationale for γ-globin gene addition is based on the observation that fetal hemoglobin (HbF, α2γ2) is a more potent antisickling hemoglobin as compared with adult hemoglobin (α2β2).52 It has long been appreciated that SCD patients with increased levels of HbF have an attenuated clinical course.53,54 There appears to be a dose-response relationship, where even the upper quartile of SCD patients with HbF levels >8.6% have extended survival, Arab-Indian haplotype patients with ∼20% HbF have mild clinical phenotypes, and sickle hemoglobin/hereditary persistence of HbF patients with ∼30% HbF are essentially free of disease manifestations. Therefore, gene addition with γ-globin may lead to a therapeutic effect with a relatively lower requirement for gene expression as compared with β-globin.
Several early configurations of γ-globin vectors, like contemporaneous β-globin vectors, were prone to low erythroid expression, genetic recombination, expression variegation, and silencing despite several different designs.29,55 Newer-generation lentiviral vectors carrying γ-globin cassettes have been described,22,56,57 including 1 with γ-globin coding sequences and β-globin regulatory elements which could effectively correct the Berkeley SCD mouse model following reduced-intensity conditioning.58,59
Several groups have developed antisickling globin vectors that express modified β-globin capable of conferring resistance to sickle hemoglobin polymerization. An advantage of these vectors is the ease with which the contribution of various globin chains can be assessed. Because the erythropoietic stress associated with HSC transplant may lead to elevated endogenous γ-globin expression, the capacity to distinguish exogenous from endogenous globin chains facilitates evaluation of gene addition efficacy. Synthetic β-globin variants with mutations affecting axial and lateral contacts in the sickle fiber show that substitutions such as E22A and T87Q confer potent antisickling activity.60 Expression of the T87Q mutation alone from a lentiviral vector resulted in pancellular, erythroid-specific expression in a murine model of SCD at levels capable of correcting the disease pathophysiology.51 Lentiviral expression of a triple-mutant β-globin (G16D, E22A, and T87Q) with increased affinity for α-globin subunits (so-called βAS3) also corrected SCD in a murine model.61,62 An insulated βAS3 SIN lentiviral vector (βAS3-FB) efficiently transduces human SCD patient bone marrow CD34+ cells and directs exogenous globin levels within an anticipated therapeutic range in in vitro erythroid differentiation and xenograft transplant models.63
A comparison of the βAS3-FB vector and the V5m3-400 vector (a γ-globin–expressing lentiviral vector) found equivalent antisickling hemoglobin levels and phenotypic RBC correction, suggesting that either vector might be suitable for clinical development.64 This type of head-to-head comparison is an important example of collaborative effort. A limitation within the gene therapy field has been that individual groups often take different approaches in terms of vector, gene cargo, regulatory elements, insulator elements, viral production, cellular transduction, experimental models, and other variables. Although in the end this may help accelerate innovation, it complicates isolating the effects of individual parameters.
Current SCD gene therapy clinical trials
Three groups have open clinical trials for SCD gene therapy registered on clinicaltrials.gov65 (Table 1; Figure 1). Bluebird Bio, a biotechnology company, is the first to treat a SCD patient with gene therapy. Their vector, LentiGlobin BB305,66 expresses an antisickling β-globin (T87Q). The vector utilizes modified LCR and β-globin promoter regulatory elements and does not include insulator sequences. Treatment of the first SCD gene therapy patient, a 13-year-old subject, resulted in a vector copy number of 2.4 copies per peripheral blood leukocyte and 24% antisickling (exogenous) hemoglobin 4.5 months following autologous hematopoietic transplantation with transduced CD34+ cells with no adverse events reported.67 Two other trials are open: 1 at the University of California, Los Angeles63,64 and the other at Cincinnati Children’s Hospital Medical Center,14,65 though neither group has yet reported patient results.
Open sickle cell gene therapy trials
| Group . | Vector* . | Conditioning . | Enrollment . | ClinicalTrials.gov identifier . | Status . | |
|---|---|---|---|---|---|---|
| Gene cassette . | Insulator . | |||||
| Bluebird Bio | βA-T87Q-globin | None | Busulfan: 12.8 mg/kg IV, pK adjusted | Ages 5-37 y, up to 7 subjects; adult, up to 8 subjects | NCT02140554; NCT02151526 | Ongoing: at least 1 patient treated67 |
| University of California, Los Angeles | βAS3-globin | FB† | Busulfan: 12.8 mg/kg IV, pK adjusted | Adult, up to 6 subjects | NCT02247843 | Open |
| Cincinnati Children’s Hospital Medical Center | γ-globin | None | Melphalan: 140 mg/m2 | Adult to age 35 y, up to 10 subjects | NCT02186418 | Open |
| Group . | Vector* . | Conditioning . | Enrollment . | ClinicalTrials.gov identifier . | Status . | |
|---|---|---|---|---|---|---|
| Gene cassette . | Insulator . | |||||
| Bluebird Bio | βA-T87Q-globin | None | Busulfan: 12.8 mg/kg IV, pK adjusted | Ages 5-37 y, up to 7 subjects; adult, up to 8 subjects | NCT02140554; NCT02151526 | Ongoing: at least 1 patient treated67 |
| University of California, Los Angeles | βAS3-globin | FB† | Busulfan: 12.8 mg/kg IV, pK adjusted | Adult, up to 6 subjects | NCT02247843 | Open |
| Cincinnati Children’s Hospital Medical Center | γ-globin | None | Melphalan: 140 mg/m2 | Adult to age 35 y, up to 10 subjects | NCT02186418 | Open |
All vectors described are SIN lentiviruses.
Enhancer blocker only.
With these trials now in the clinic, it is imperative to establish robust means for assessing efficacy. Although vector marking, transgene expression, and hematopoietic reconstitution have been established as important measures in other lentiviral-based gene therapy trials,68 determinants of success for SCD gene therapy should also include objective clinical criteria, such as survival, hospitalizations, painful crises, and other SCD-associated events (such as acute chest syndrome, stroke, etc) as well as patient-reported outcomes. Although these clinical outcomes will require evaluation of numerous patients over an extended period of time, they will constitute the ultimate evidence for safety and efficacy of gene therapy.
Genome engineering and other novel strategies
The advent of genome engineering has expanded possible genetic therapy options for many hematopoietic diseases. Given their prevalence and severity, the hemoglobinopathies have attracted considerable attention. Targeted nucleases, such as zinc-finger nucleases (ZFNs), transcription-activator like effector nucleases (TALENs), meganucleases, and the CRISPR (clustered, regularly interspaced palindromic repeats)-associated nuclease Cas9, each have programmable DNA-binding modules that allow introduction of site-specific double-strand breaks into the human genome69-71 (Table 2). Unlike the other nucleases, Cas9 utilizes RNA to guide target sequence recognition. Following cleavage, subsequent endogenous eukaryotic repair mechanisms may be simplified into 2 major pathways: nonhomologous end joining (NHEJ) and homology-directed repair (HDR). The ability to direct either of these pathways to produce predictable modifications could be of therapeutic relevance for SCD69,72,73 (Figure 2). Unlike gene addition strategies, genome engineering approaches only rely on a transient ex vivo intervention and do not result in permanent insertion of foreign DNA into the genome.
Targeted nucleases
| Nuclease . | DNA-binding domain . | Active configuration . | Cleaving enzyme; break characteristic . | Target range . |
|---|---|---|---|---|
| Homing endonucleases (meganucleases) | 5 families: LAGLIDADG, GIY-YIG, HNH, His-Cys box, and PD- (D/E)XK | Homodimeric or monomeric | 3′ overhang | 14-40 bases |
| ZFN | Zinc-finger motif | Protein dimer | FokI; 5′ overhang | 9-18 bases per monomer |
| TALEN | Repeat variable diresidue | Protein dimer | FokI; 5′ overhang | 12-31 bases per monomer |
| CRISPR/Cas9* | CRISPR-RNA and trans-activating RNA | RNA/protein complex | Cas9; Blunt | 17-20 bases |
| Nuclease . | DNA-binding domain . | Active configuration . | Cleaving enzyme; break characteristic . | Target range . |
|---|---|---|---|---|
| Homing endonucleases (meganucleases) | 5 families: LAGLIDADG, GIY-YIG, HNH, His-Cys box, and PD- (D/E)XK | Homodimeric or monomeric | 3′ overhang | 14-40 bases |
| ZFN | Zinc-finger motif | Protein dimer | FokI; 5′ overhang | 9-18 bases per monomer |
| TALEN | Repeat variable diresidue | Protein dimer | FokI; 5′ overhang | 12-31 bases per monomer |
| CRISPR/Cas9* | CRISPR-RNA and trans-activating RNA | RNA/protein complex | Cas9; Blunt | 17-20 bases |
Characteristics listed here describe Streptococcus pyogenes Cas9. Other Cas9 variants and orthologs have related but not identical properties.
Strategies for gene therapy for SCD: schematic overview of various approaches for correcting the sickle phenotype via gene therapy. Gene correction: targeted genome engineering leads to correction of the sickle mutation such that βS is repaired as βA. HbF induction: multiple strategies for induction of γ-globin expression include shRNA-mediated knockdown of BCL11A, targeted disruption of the +58 DNase I HS site in the BCL11A erythroid-specific enhancer, and forced chromatin looping to promote association of the β-globin LCR with the γ-globin genes. Gene addition: integrating lentiviral vector carrying a β-globin, γ-globin, or antisickling β-globin cassette. Ldb1, transcription factor; ZF/SA, zinc-finger self-association domain.
Strategies for gene therapy for SCD: schematic overview of various approaches for correcting the sickle phenotype via gene therapy. Gene correction: targeted genome engineering leads to correction of the sickle mutation such that βS is repaired as βA. HbF induction: multiple strategies for induction of γ-globin expression include shRNA-mediated knockdown of BCL11A, targeted disruption of the +58 DNase I HS site in the BCL11A erythroid-specific enhancer, and forced chromatin looping to promote association of the β-globin LCR with the γ-globin genes. Gene addition: integrating lentiviral vector carrying a β-globin, γ-globin, or antisickling β-globin cassette. Ldb1, transcription factor; ZF/SA, zinc-finger self-association domain.
Gene correction
Site-specific gene correction of the causal sickle β-globin mutation in HSCs would seem the most straightforward strategy for gene therapy. In principle, this approach only requires transient delivery of nuclease and repair template to achieve correction, thereby avoiding introduction of foreign DNA and associated risk of insertional oncogenesis as occurs with viral vectors. In addition, direct correction of the sickle mutation has the advantage of preserving endogenous globin regulatory mechanisms to achieve the appropriate balance of globin chains during erythropoiesis.
Correction of the sickle mutation by targeted nucleases followed by HDR in various cell types has been demonstrated,74,75 including reports of correction of induced pluripotent stem cells from both mice and humans.75-78 In addition, oligonucleotide-based gene therapy strategies, such as triplex-forming peptide nucleic acids which rely on HDR but not on the initial formation of a double-stranded break, have achieved low-frequency correction of the SCD mutation.79-82 Although these approaches offer the possibility to determine genome modification specificity on a clonal level, derivation of functional HSCs from pluripotent cells remains a great challenge.14,83,84 Recently, correction in human HSCs was reported. However, the rates of correction in long-term HSCs were well below levels necessary for therapeutic benefit.85 A similar finding of preferential utilization of NHEJ in HSCs (despite relatively robust HDR repair in unfractionated CD34+ hematopoietic stem and progenitor cells) has been observed in experiments attempting to correct the SCID-X1 mutation in human HSCs.86 A simple explanation of this observation may be that the HDR pathway is restricted to the S and G2 phases of the cell cycle when sister chromatids are available as donor repair template sequences. In contrast, HSCs, which are largely quiescent cells, rely mainly on NHEJ.87
Reactivation of fetal hemoglobin
Although technical advances may be required to realize therapeutic HDR in HSCs, NHEJ appears to be a robust repair pathway in these cells. A clinical trial of genetic disruption by NHEJ in T cells has already been conducted using ZFN targeting of CCR5 in HIV-infected subjects demonstrating safety and genetic efficacy.88 A growing understanding of mechanisms of developmental globin gene regulation suggests alternate strategies that could be appropriate for genetic disruption rather than repair.89 These approaches are neither gene addition nor gene correction per se but rather target a modifier of disease severity. Although this strategy of modifying a modifier might seem somewhat indirect, it actually capitalizes on knowledge of naturally occurring protective mechanisms in SCD (ie, elevated HbF level).
Genome-wide association studies have implicated 3 key loci (BCL11A, the intergenic region between HBS1L and MYB, and the β-globin cluster itself) in regulation of HbF level.90,91 BCL11A is critically required for HbF repression in primary human erythroid cells and in transgenic mice.92-94 The fact that RBC production remains ostensibly normal in Bcl11a knockouts is surprising for a critical transcription factor and distinguishes BCL11A from many other potential targets that have broad requirements in erythropoiesis. Erythroid-specific loss of BCL11A in mouse models of SCD is sufficient to reverse the hematologic and pathologic manifestations of disease, thereby validating BCL11A as a therapeutic target.94 As BCL11A also has important roles in B-cell and dendritic cell development, its complete knockout in HSCs could impair production of immune cells.95-97 Furthermore, BCL11A is expressed in HSCs and early progenitor cells, suggesting it might play additional roles in homeostasis of the hematopoietic system.96 The identification of the intronic erythroid-specific BCL11A enhancer as the site of common genetic variants associated with HbF level has suggested that disruption of the erythroid enhancer could circumvent negative effects of impairment of BCL11A in nonerythroid contexts by only affecting its expression in erythroid precursors.98 Recently, a systematic CRISPR screen saturating the DNase I HS sites with guide RNAs identified critical minimal sequences within the +58 DHS required for erythroid expression of BCL11A and adult-stage repression of HbF. Single cleavages targeting these crucial enhancer sequences were found to be sufficient for robust HbF induction in primary human erythroid precursors.99 A complementary approach targeting DNase I footprints within the BCL11A erythroid enhancer revealed similar critical minimal sequences that could be disrupted by ZFNs.100 Production of single cleavages by targeted nucleases is an appealing genome engineering strategy in that it is relatively simple and takes advantage of the endogenous error-prone NHEJ repair mechanism that is robust in HSCs.
An alternative approach to BCL11A knockout is reduction of BCL11A expression below a critical threshold through RNA interference.92,101 A novel short hairpin RNA (shRNA) vector was recently reported, capable of potent BCL11A knockdown in human erythroid precursors. This vector used a Pol II microRNA architecture, suggesting that knockdown controlled by lineage-specific regulatory elements could be a strategy for restricting gene inhibition to erythroid cells.102 Similar to gene addition approaches, this strategy would rely on both HSC delivery by integrating vectors and sustained erythroid expression, in this case of the inhibitory RNA.
The ability to program DNA-binding specificity of synthetic factors also allows for targeted gene regulation.103 Expression of the self-association domain of Ldb1 (a transcription factor involved in chromatin looping of the LCR) linked to artificial zinc fingers recognizing the γ-globin promoter in adult human erythroblasts resulted in robust increases in γ-globin.104 These experiments suggest that expression of such a construct could lead to therapeutic increases in γ-globin expression at the expense of βS-globin in SCD. By necessity, this strategy would rely on long-term expression from integrating vectors in HSCs. Perhaps the requirements for lower-level expression could be a safety advantage over classic globin gene addition. The potency of this effect, the continuous expression of foreign protein, as well as specificity for globin gene expression as compared with other transcriptional impacts, are important issues to be considered before further clinical development.
Combined approaches (globin gene addition plus HbF induction) for SCD gene therapy have also been explored.105,106 These multipronged approaches might prove superior to single modality vectors, but their effects and potential off-target issues would need to be investigated thoroughly. As knowledge expands of mechanisms repressing HbF in the adult stage, additional targets may emerge. Although not explicitly discussed here, similar strategies as above could be contemplated to target other HbF repressors or to mimic naturally occurring variation at the β-globin cluster associated with hereditary persistence of HbF.107-109
Keys to successful gene therapy
Irrespective of which gene therapy strategy is considered for SCD, there are several common critical factors that will determine success or failure in the clinical setting.
Adequate cell dose
Autologous engraftment is highly dependent on the dose of CD34+ cells.110 Mobilization of CD34+ cells by granulocyte colony-stimulating factor treatment followed by peripheral blood apheresis is most commonly used in conventional bone marrow transplant and in hematopoietic cell gene therapy. However, granulocyte colony-stimulating factor mobilization has been associated with severe adverse events and even mortality in SCD patients.111,112 Thus, gene therapy approaches for SCD have focused on relatively more invasive bone marrow harvest as cell source of CD34+ cells. Even this procedure is not completely without risk, given anesthesia dangers in SCD. Use of plerixafor for CD34+ cell mobilization for gene therapy has been widely investigated, including in β-thalassemia patients. Clinical trials for plerixafor mobilization in SCD are planned but results have not yet been reported.113 Safe and effective collection of HSCs will be essential for the subsequent repopulation of the bone marrow.
Assessment of true HSCs and their modification
CD34+ cells, either mobilized or harvested from marrow, are a mixture of many progenitors and few true HSCs. A current challenge in the field is that it is not yet possible to determine the fraction of modified HSCs based on the level of marking of unfractionated CD34+ cells. The current laboratory standard is to perform engraftment of immunodeficient mice, which is both time-consuming and costly. Of greater concern is that these xenograft systems do not fully support human hematopoiesis (and in particular do not support human erythropoiesis). Secondary transplant can be very difficult to achieve, limiting the ability to truly test long-term self-renewal activity. A promising approach is to test modification within subpopulations of CD34+ cells enriched for HSCs as defined by immunophenotype, such as by expression of CD38, CD133, CD90, and CD45RA,114-117 though this may require short-term culture.118 Targeted transfer into subpopulations of CD34+ cells enriched for HSCs have also been investigated using a vector with tropism for CD133.119 Efforts at in vitro expansion of human HSCs, while preserving their stem cell qualities, might advance efforts at HSC modification.120-123 Careful correlation of laboratory parameters of the modified graft with clinical outcomes in gene therapy subjects may eventually inform optimal surrogate markers to predict HSC activity.
High levels of modified-HSC engraftment
Another important consideration for SCD gene therapy is the fraction of modified cells as compared with the entire hematopoietic compartment. Based on allogeneic HSC transplant for SCD patients, stable donor chimerism of 10% to 30% may provide significant clinical improvement.6,124-126 These results reflect that the survival advantage of nonsickle cells both at the level of erythrocytes (reduced hemolysis) and erythroblasts (reduced ineffective erythropoiesis) leads to selective survival of corrected RBCs out of proportion to overall hematopoietic engraftment.127 Stable engraftment of 10% to 60% gene-modified HSCs has been observed in other clinical trials with fully cytoablative conditioning.44,45,128,129 This degree of engraftment would appear to approximate or exceed the threshold required for substantial clinical benefit. Despite the above inferences, there remains some uncertainty as to the minimal level of modification required to derive curative outcome for SCD. It will be critically important for careful evaluation of the fraction of modified cells within erythroid and nonerythroid progenitor cells in subjects of SCD gene therapies to empirically derive the quantitative relationship between genetic modification of HSCs and hematologic and clinical outcomes.
Although the degree of myeloablation has been shown to be proportional to the degree of autologous engraftment and thus to correction efficiency,68,110,130 fully myeloablative regimens with alkylating chemotherapy carry nontrivial risks to patients, especially to SCD patients who may already have experienced some disease-related end-organ compromise. A goal for the field would be to develop novel approaches to achieve engraftment while limiting preparative conditioning-related toxicity. One approach that appears promising in mouse models is transient inhibition of stem cell factor/c-Kit signaling.131-133
Safety
Independent of the type of gene therapy, safety must be paramount. For integrating viral vectors, safety evaluation focuses on insertional mutagenesis with risk for clonal dominance or cellular transformation. Clinical gene therapy trials using γ-retroviral vectors for other diseases have been accompanied unfortunately by severe adverse events due to negative effects of the integration site of the vector.129,134-136
It is estimated that successful gene therapy with current methods results in thousands of viral insertions per recipient, each insertion measured as the hematopoietic output of a uniquely marked clone.137-139 These many insertions carry an inherent risk for insertional oncogenesis or loss of appropriate gene regulation by mechanisms such as aberrant splicing.128,136 Although safety may be modulated by vector design, including the use of insulators, optimal cellular promoters, and lentiviral vectors,140,141 it may not be possible to fully eliminate risk from thousands of semirandom genomic viral integrations.
A gene therapy clinical trial for β-thalassemia using a γ-retroviral vector resulted in the outgrowth of a myeloid-biased hematopoietic clone associated with integration-mediated transcriptional activation of HMGA2 due to aberrant splicing. The affected patient achieved transfusion independence without leukemia development, so for this patient the clonal dominance may have provided some advantage in that the corrected cells were present at a high frequency.128,142 Of note, the causal cryptic 3′ splice signal was introduced by rearranged sequences from a tandem repeat of the cHS4 insulator region of the vector, turning an intended protective feature into one apparently compromising safety. The myeloid bias of the long-lived corrected hematopoietic progenitor provocatively suggests that modification of long-lived lineage-restricted progenitors (in addition to true HSCs) could prove therapeutically beneficial in certain contexts.143,144
For genome engineering approaches, evaluation of off-target mutagenesis will be important preclinical criteria prior to embarking on clinical studies. Typically these approaches have used computational predictions and/or empirically determined sites of chromatin binding to prioritize a limited set of susceptible genomic sites for analysis.145 More recently, unbiased methods have been developed that allow for genome-wide assessment of off-target mutagenesis.146-151 Cellular products for autologous HSC transplant would typically include 108 or more cells, so it is virtually impossible to measure very rare off-target mutations. The significance of modifications at most genomic sites is uncertain, and likely the vast majority of modifications would be functionally neutral. The occasional somatic mutations that accompany normal aging and environmental exposures also raise the question of baseline level of mutagenesis.152 These challenges to assaying and interpreting genetic perturbations emphasize the importance of functional readouts of safety. In the end, investigators need to maintain humility that any laboratory safety testing will likely be incompletely predictive of in vivo behavior of modified cells in humans.153 As with all novel therapeutic approaches, meticulous clinical trials will be essential to ensure the success and safety of genetic approaches to SCD.
Conclusions
Although several of the initial hurdles to SCD gene therapy appear to have been overcome, it is prudent to recognize barriers that remain. Efficient transduction of HSCs with lentiviral vectors has become increasingly reliable, but the complicated components of many globin vectors present unique challenges for production of high-titer virus capable of robust transduction. Scaling up procedures to multiple patients is a nontrivial challenge. Safety and efficacy can only be established by careful clinical trials with extended patient follow-up. Gene engineering methods are rapidly evolving and should facilitate development of “second-generation” gene therapy approaches in the coming years. After many years of preclinical laboratory investigation, gene therapy options are now on the horizon for patients with SCD.
Acknowledgments
The authors thank Marina Cavazzana, Donald Kohn, and Punam Malik for personal communications regarding open gene therapy trials, and Brian Sorrentino and Christian Brendel for helpful comments.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants T32HL007574 (M.D.H.), R01HL032259, and P01HL032262, and National Institute of Diabetes and Digestive and Kidney Diseases grants P30DK049216 (Center of Excellence in Molecular Hematology) (S.H.O.) and K08DK093705 (Career Development Award) (D.E.B.). This work was also supported by the Doris Duke Charitable Foundation, the Charles H. Hood Foundation, and the Cooley’s Anemia Foundation (D.E.B.).
Authorship
Contribution: M.D.H., S.H.O., and D.E.B. wrote and edited the manuscript.
Conflict-of-interest disclosure: D.E.B. and S.H.O. are inventors on a patent application related to therapeutic genome editing of BCL11A. D.E.B. has served as a consultant to Editas Medicine. S.H.O. was previously on the Scientific Advisory Board of Editas Medicine. D.E.B. and S.H.O. have received research support from Biogen. M.D.H. declares no competing financial interests.
Correspondence: Stuart H. Orkin, Department of Pediatrics, Harvard Medical, Harvard Stem Cell Institute, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; email: orkin@bloodgroup.tch.harvard.edu.