Abstract
With advances in brain imaging and completion of randomized clinical trials (RCTs) for primary and secondary stroke prevention, the natural history of central nervous system (CNS) complications in sickle cell disease (SCD) is evolving. In order of current prevalence, the primary CNS complications include silent cerebral infarcts (39% by 18 years), headache (both acute and chronic: 36% in children with sickle cell anemia [SCA]), ischemic stroke (as low as 1% in children with SCA with effective screening and prophylaxis, but ∼11% in children with SCA without screening), and hemorrhagic stroke in children and adults with SCA (3% and 10%, respectively). In high-income countries, RCTs (Stroke Prevention in Sickle Cell Anemia [STOP], STOP II) have demonstrated that regular blood transfusion therapy (typically monthly) achieves primary stroke prevention in children with SCA and high transcranial Doppler (TCD) velocities; after at least a year, hydroxycarbamide may be substituted (TCD With Transfusions Changing to Hydroxyurea [TWiTCH]). Also in high-income countries, RCTs have demonstrated that regular blood transfusion is the optimal current therapy for secondary prevention of infarcts for children with SCA and strokes (Stroke With Transfusions Changing to Hydroxyurea [SWiTCH]) or silent cerebral infarcts (Silent Infarct Transfusion [SIT] Trial). For adults with SCD, CNS complications continue to be a major cause of morbidity and mortality, with no evidence-based strategy for prevention.
Introduction
With recent advances in medical treatment, the natural history of sickle cell disease (SCD) continues to evolve as morbidity and mortality fall. In the last 40 years, significant clinical research efforts have been focused on preventing initial and subsequent central nervous system (CNS) injuries. In children and adults with SCD, from the 1970s until 2010, ∼75% of the infarcts were ischemic and the remainder hemorrhagic.1,2 In the 1990s, the prevalence of the first transient ischemic attack, infarctive or hemorrhagic stroke in children with sickle cell anemia (SCA) younger than 19 years was 11% and 24% by 45 years for adults with SCA.3 Since the 1990s, 4 major randomized trials to prevent CNS injuries have been completed, providing evidence-based guidelines for primary and secondary stroke prevention in children with SCA.4-8
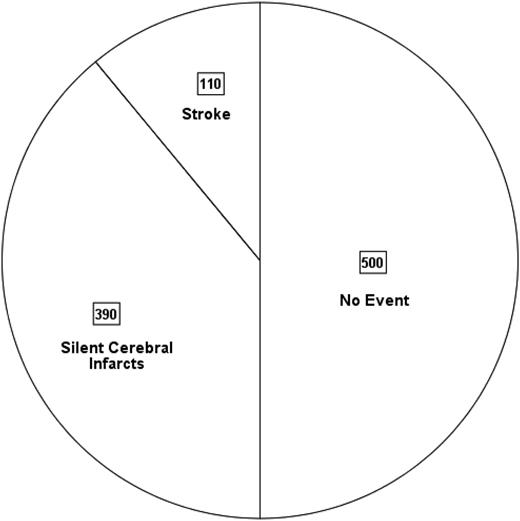
Unfortunately, in sub-Saharan African countries and India, where >90% of the children with SCA are born,9 there are no evidence-based primary and secondary stroke-prevention strategies. Thus, before their 18th birthday, ∼50% of the children with SCA will have either an overt or silent cerebral infarct (SCI) (Figure 1).
Pie chart depicting the prevalence of overt strokes and SCIs in 1000 children born with SCA followed for 18 years in sub-Saharan African countries and India, where primary prevention is not routinely practiced. This is based on an assumption that 11%3 will have a stroke and 39% will have SCIs prior to the 18th birthday22 ; see text for details.
Pie chart depicting the prevalence of overt strokes and SCIs in 1000 children born with SCA followed for 18 years in sub-Saharan African countries and India, where primary prevention is not routinely practiced. This is based on an assumption that 11%3 will have a stroke and 39% will have SCIs prior to the 18th birthday22 ; see text for details.
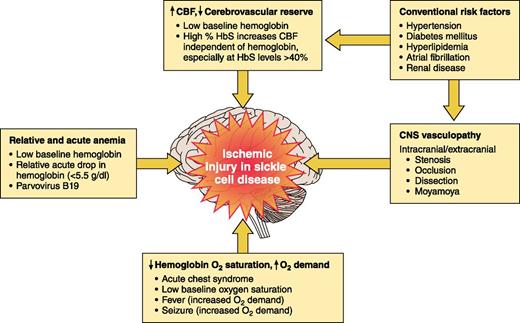
The pathophysiology of ischemic stroke and cerebral hemorrhage in SCD is not well defined10 but over the last 3 decades, we have begun to understand more about clinical risk factors and potential mechanisms of brain injury (Figure 2). At least 6 risk factors are associated with cerebral ischemic events in SCD:
Low oxygen content associated with
Presence of cerebral vasculopathy compromising cerebral blood flow (CBF), acting synergistically with the compensatory increase in CBF secondary to
Acute infection with fever14 increasing cerebral metabolic demands.
Cardiovascular risk factors as in the general population: hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, and renal disease.2
Presence of a prior cerebral infarct with the greatest risk being within 2 to 3 years of infarct occurrence, regardless of treatment.1,13
Rapid increases in hemoglobin levels, typically >12 g/dL, with either autotransfusion from splenic or liver sequestration16 or blood transfusion therapy.17
Risk factors for ischemic stroke in SCA. Established risk factors for ischemic injury of the brain in children and adults with SCA.
Risk factors for ischemic stroke in SCA. Established risk factors for ischemic injury of the brain in children and adults with SCA.
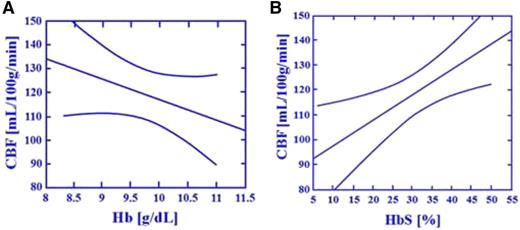
The critical relationship between cerebral blood flow and HbS levels in individuals with SCA. The study conducted by Hurlet-Jensen et al15 describes the unique relationship between hemoglobin (Hb) and HbS levels and CBF in individuals with SCA. As Hb levels increase, CBF decreases (r = −0.68, P = .006). As HbS levels increase, CBF increases (r = .080, P = .0003). In a stepwise multiple regression equation with Hb and HbS, only HbS was accepted and predictive of CBF (r = 0.70, P = .01). The figure depicts regression lines with 95% CI describing the relationship between Hb, HbS, and CBF. (A-B) Effect of total Hb and HbS on regional CBF measured by Xe inhalation: transfusion therapy.
The critical relationship between cerebral blood flow and HbS levels in individuals with SCA. The study conducted by Hurlet-Jensen et al15 describes the unique relationship between hemoglobin (Hb) and HbS levels and CBF in individuals with SCA. As Hb levels increase, CBF decreases (r = −0.68, P = .006). As HbS levels increase, CBF increases (r = .080, P = .0003). In a stepwise multiple regression equation with Hb and HbS, only HbS was accepted and predictive of CBF (r = 0.70, P = .01). The figure depicts regression lines with 95% CI describing the relationship between Hb, HbS, and CBF. (A-B) Effect of total Hb and HbS on regional CBF measured by Xe inhalation: transfusion therapy.
Adults with SCD have different risk factors for strokes and different prevalence of ischemic and hemorrhagic stroke than children with SCD. In a large population study, the biggest risk factor for strokes in children with SCD was hypertension3 ; whereas, in adults with SCD, the biggest risk factors included not only hypertension, but also diabetes mellitus, hyperlipidemia, atrial fibrillation, and renal disease.18 The determinants of cognition in adults with SCD are not well documented. In asymptomatic adults with SCA undergoing cognitive testing, performance IQ was not associated with sex, white blood cell count, platelet count, or levels of hemoglobin F, lactate dehydrogenase, or hemoglobin.19 This review will initially focus on the epidemiology of the most common CNS complications, primarily in SCA, and then will review the evidence for primary and secondary stroke prevention in high- and low-income countries.
SCIs are common in SCA
SCI is the most common permanent neurological injury in children with SCA, and probably in adults as well. The only current strategy to detect an SCI is a 30- to 60-minute magnetic resonance imaging (MRI) of the brain. The most complete definition of an SCI has both a neuroimaging component and an assessment by a neurologist.7 The imaging component requires a signal abnormality that is at least 3 mm in 1 dimension and that is visible in 2 planes on fluid-attenuated inversion recovery T2-weighted images. The neurology component includes a normal neurologic examination or an abnormality on examination that could not be explained by the location of the brain lesion or lesions.7 The neurologic evaluation by a neurologist is critical because individuals who are previously labeled as having an SCI may in fact have an undetected stroke, a higher risk category for future neurologic morbidity.20
SCIs are common in infants and preschool children. Approximately 25% of children with SCA will have an infarct prior to their sixth birthday21 and 39% by their 18th birthday,22 with no evidence that the number of children with new SCI plateaus through 20 years of age (Figure 421-25 ). Well-established risk factors for SCI include low baseline hemoglobin,27 particularly before age 3 years,22 relative hypertension,27 acute anemia events,13,14,22 and evidence of cerebrovascular disease on intracranial28,29 and extracranial22 magnetic resonance angiography (MRA). Unfortunately, the presence of multiple SCI risk factors together or independently has not led to accurate prediction of SCI. In children with SCD and SCI who do not have either SCA or hemoglobin S-β0 thalassemia (entry criteria for the Silent Infarct Transfusion [SIT] Trial), management should be based on an individual basis until large multicenter studies are completed to identify optimal management strategies for this population. A more detailed review on the natural history, detection, and mimics of SCI in SCD has been recently published.30
Prevalence of SCIs in children with SCA. The figure displays the cumulative prevalence of SCIs in children with SCA based on 4 cross-sectional studies21,23-25 and 1 longitudinal study.22 The cumulative prevalence of SCIs strongly suggests that the incidence rates of SCIs dose not plateau up to 18 years of age.
Prevalence of SCIs in children with SCA. The figure displays the cumulative prevalence of SCIs in children with SCA based on 4 cross-sectional studies21,23-25 and 1 longitudinal study.22 The cumulative prevalence of SCIs strongly suggests that the incidence rates of SCIs dose not plateau up to 18 years of age.
Ischemic lesions of the brain may be temporary or evolve to infarcts
Children with SCA are at increased risk for cerebral lesions likely to be ischemic, but not necessarily progressing to infarction. Diffusion weighted imaging (DWI) allows detection of acute ischemic events that occurred within the previous 10 days. As part of the screening protocol to detect SCI in the SIT Trial,7 children (n = 652) received MRI of the brain with DWI.31 In this asymptomatic group of children, the incidence of acute silent cerebral ischemic events was 47.3 per 100 patient-years (95% confidence interval [CI], 22.7-87.2),31 nearly 7 times higher than the anticipated incidence of infarct recurrence in children with preexisting SCI,32 but only 1 of 2 children with follow-up MRI of the brain had an infarct. These results strongly suggest that brain ischemia in children with SCA is common and potentially reversible. Undoubtedly, as MRI scanners continue to improve in magnet strength, resulting in improvement in detecting cerebral infarcts, the prevalence of ischemic brain injury and SCI will also increase33 and the epidemiology of both injuries will change. However, MRI of the brain in children and adults with SCD cannot replace a thorough neurologic examination because in the general population, as many as 33% of the individuals with confirmed nondisabling strokes have negative DWI MRI of the brain.34
Acute and chronic headaches are challenging to manage in SCD
Headache is one of the most common neurologic symptoms in SCD, both acutely35 and chronically.36 In acute headache, hemorrhagic stroke (see supplemental Figure 1A-B, available on the Blood Web site) must be considered. In the Hines study of acute care visits by children with SCA, headache was the chief complaint in 3.8% (102 of 2685).35 In this group of children presenting with acute headaches, 6.9% (7 of 102) had clinically significant CNS pathology requiring immediate medical management (3 venous thrombosis, 3 intracranial hemorrhage, 1 ischemic stroke).35 Acute CNS events associated with headaches were more likely with older age, history of stroke, transient ischemic attack or seizure, neurologic symptoms, and focal neurologic findings.35 Unfortunately, no clinical features were identified to clearly separate those who did or did not require immediate medical management. Neuroimaging was performed in 42.2% of visits, and acute CNS events were identified in 16.3% of studies. Distinguishing whether the headache is chronic or a new symptom requiring emergent imaging is critical. The decision to obtain an MRI of the brain involves eliciting a thorough personal and family history of chronic migrainous symptoms and careful examination of level of consciousness, vision, and any focal signs. For nocturnal or early morning headaches, space-occupying lesion or idiopathic intracranial hypertension37 must be excluded. For these reasons, we believe a neurologist should be consulted for severe new-onset acute headaches.
MRI is not essential for children with SCA and chronic headache, including migraines. In the largest retrospective cross-sectional study in children with SCA (n = 872), recurrent headaches (36.1%) and migraines (15.1%) were both common, but neither was associated with SCI.38 Referral to a neurologist is advised for assessment of chronic headache and management with diet,39 psychological support, and prophylactic medication (pizotifen, propranolol, topiramate, or valproic acid). We do not recommend using triptans for acute migrainous headaches because of the risk of cerebral ischemia. Although headache is common, unfortunately, no specific evidence-based guidelines for management have been developed for SCD.
Hemorrhagic stroke and aneurysms are more common in adults
Intracerebral, intraventricular, subarachnoid, subdural, and extradural hemorrhages have all been described in patients with SCD (see supplemental Figure 1 A-B).40 Hemorrhage, typically subarachnoid, has the highest incidence in young adults (20-30 years),3,41 but is not uncommon in children.3 Eleven of 325 children with SCD (3.4%) followed at the University of Illinois between 1975 and 1989 had subarachnoid hemorrhage.42 In a French cohort, 2.8% of children with SCD (7 of 251) had either an intracranial hemorrhage or an unruptured aneurysm.43,44 Risk factors for cerebral hemorrhage are poorly defined, but include hypertension associated with the use of corticosteroids40 or phenylephrine,45 recent transfusion,40 splenic sequestration,46 and hyperviscosity associated with higher hemoglobin levels.16
Cerebral hemorrhage in adults is commonly related to aneurysm formation (see supplemental Figure 1C-D).47 In a large study (n = 709), the prevalence of aneurysm was 1.2% and 10.8% in children and adults with SCD, respectively.48 The aneurysms which ruptured were typically relatively small (2-9 mm)48 and located at the bifurcations of major vessels.47,48
Individuals with SCD and intracranial hemorrhage require immediate transfer to a tertiary hospital where a multidisciplinary team includes a neurosurgeon with a vascular interventional neuroradiologist, neurologist, and hematologist. We are unaware of any contraindication to management of hemorrhage as for the general population.48
Extradural and subdural intracranial hemorrhage may occur without trauma
One of the most underrecognized complications of SCD is extradural or subdural hematoma in the absence of significant head trauma,49,50 probably related to hypervascular areas of bone,51 bone infarction,52-54 or venous thrombosis10 (see supplemental Figure 1B). Bony infarction in the orbit may be associated with periosteal hemorrhage and compressive optic neuropathy with risk of visual loss, whereas subgaleal hemorrhage, which may be substantial as there is a large potential space, may also occur.55 These lesions are typically managed with appropriate supportive care and transfusion, but may require surgery.56
Seizures and epilepsy must be distinguished and managed appropriately
In the era prior to implementing a primary stroke-prevention strategy, between 7% and 10% of individuals with SCD experienced at least 1 seizure.57,58 In the Jamaican cohort Study of Sickle Cell Disease, the 5-year cumulative incidence of febrile convulsions was 2.2%.58 Even in the context of a febrile or systemic illness, in patients with SCD, we recommend brain MRI for first seizure to exclude the possibility of a potentially treatable underlying cause, such as arterial ischemic stroke, sinovenous thrombosis, reversible posterior encephalopathy syndrome, fat embolism, or cerebral abscess (see supplemental Figure 2A-H). A history of seizures has been associated with SCI.59
Epilepsy (recurrent seizures) is 2 to 3 times more common in individuals with SCD than in the general population, and is associated with earlier death.58,60 The majority of patients have focal abnormality on neuroimaging or electroencephalography61 and cerebrovascular disease (see supplemental Figure 3A-D).62 Given the high rate of moyamoya in individuals with SCD, we do not recommend hyperventilation during electroencephalography because of the risk of ischemia.63
CNS vasculopathy is associated with stroke and SCI
Stenosis and occlusion of the large arteries of the circle of Willis have been recognized in SCD for >40 years on pathology64 and contrast arteriography.65 More recently, less invasive techniques, including transcranial Doppler (TCD) ultrasound and MRA, have been used to detect and follow any improvement or progression, but they do not directly visualize the vessel diameter or wall and interpretation requires considerable skill. Higher hemoglobin levels provide better MRA images.
In the largest study to date (n = 516), MRA-defined vasculopathy occurred in 10.3% of asymptomatic children with SCA without prior stroke.28 The presence of MRA-defined cerebral vasculopathy was associated with SCI, although the vast majority (84%) of children with SCA and SCI did not have MRA-defined vasculopathy.28 Among children with SCA and strokes, MRA is abnormal in 50% to 60% of children.66,67 Among children with SCA and progressive vasculopathy, 100% had evidence of new cerebral infarct within 5.5 years.66
Despite the routine use of TCD to identify children with SCA at risk for overt strokes, several nuances of its use exist. First, there are 2 different methods for imaging the intracranial vessels: the imaging and nonimaging techniques.68 Both are routinely used, with most pediatric radiology departments preferring the imaging technique and most research studies using the nonimaging technique. The relative benefits and challenges with each technique are discussed in depth elsewhere.68 The most important point is that the threshold for transfusion used in the nonimaging technique is a time-averaged maximum velocity of 200 cm per second, which is well defined and may also be appropriate for the imaging technique,69 whereas other investigators in a clinical trial setting have lowered the threshold of time-averaged maximum velocity (TAMX in imaging machine) ≥185 cm per second.7 For imaging TCD, until a rigorous head-to-head study is done comparing these 2 techniques, we prefer the more conservative strategy, namely to lower the threshold for treatment to 180 to 185 cm per second.
Another subtlety in the use of TCD is that in ∼10% of the children, velocities are low or absent70,71 perhaps related to vasculopathies such as moyamoya or extracranial vasculopathy.72 However, MRA-defined vasculopathy without strokes has not been demonstrated in a multicenter study to predict future strokes or SCI and cannot be reliably applied as a substitute for the lack of a TCD measurement to stratify children with SCA into high- or low-risk future stroke groups.
Treatment
In children with SCA and SCIs, regular blood transfusion decreases infarct recurrence
No strategy has been established for primary SCI prevention. The only current strategy is to prevent infarct recurrence in children with SCI. Despite the importance of detecting SCI because of the high rate of future strokes73 and SCIs,32 as well as a drop of a full-scale IQ loss of 5 points74 (Figure 5), all of the studies to date have been from high-income countries because of the expense of performing MRI of the brain.
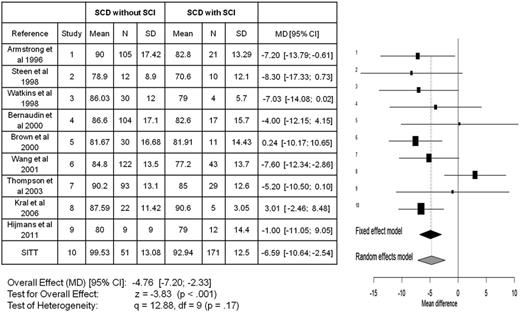
Meta-analyses for all studies in children with SCA that included full-scale IQ for those with and without SCIs. The meta-analyses include a total of 10 publications7,86-94 comparing the mean difference in full-scale IQ between those children with SCA with and without SCIs. The x-axis reflects the mean full-scale IQ difference between those with and without an SCI. The horizontal lines represent the upper and lower boundaries of the 95% CI. If the 95% CI overlaps zero or crosses the zero threshold then no statistical differences were observed in that study. The black and gray diamonds represent the results of the fixed and random-effect models. The edges of the diamonds represent the 95% CI of the meta-analyses for the fixed and random-effect models. df, degree of freedom; MD, mean difference; SD, standard deviation. Adapted from King et al.26
Meta-analyses for all studies in children with SCA that included full-scale IQ for those with and without SCIs. The meta-analyses include a total of 10 publications7,86-94 comparing the mean difference in full-scale IQ between those children with SCA with and without SCIs. The x-axis reflects the mean full-scale IQ difference between those with and without an SCI. The horizontal lines represent the upper and lower boundaries of the 95% CI. If the 95% CI overlaps zero or crosses the zero threshold then no statistical differences were observed in that study. The black and gray diamonds represent the results of the fixed and random-effect models. The edges of the diamonds represent the 95% CI of the meta-analyses for the fixed and random-effect models. df, degree of freedom; MD, mean difference; SD, standard deviation. Adapted from King et al.26
The SIT Trial (NCT00072761) was the only trial designed to determine whether blood transfusion therapy can prevent progression of infarct recurrence (stroke or SCI) in children with SCA and preexisting SCI. Participants included children with SCA (5-15 years of age) and SCIs. In the randomized clinical trial (RCT), 196 children with SCA and SCI were randomly allocated to receive either observation (standard therapy) or regular blood transfusion (experimental therapy) for 36 months. In participants receiving regular blood transfusion, there was 58% relative risk reduction in cerebral infarct recurrence (stroke or new or progressive SCI) when compared with the children in the observation arm.7 When compared with observation, the benefit of blood transfusion therapy included an improvement of overall quality of life,75 as well as a statistically significant decrease in the incidence of priapism, new-onset symptomatic avascular necrosis of the hip, severe vaso-occlusive pain events that resulted in hospitalization and acute chest syndrome.7 The number of children with SCA and SCI who needed to be transfused to prevent 1 recurrent infarct was 13.7 However, the benefit of blood transfusion therapy to prevent infarct recurrence was incomplete. Some children in the transfusion therapy arm went on to develop infarct recurrence (Figure 6). Thus, the high burden of regular blood transfusion coupled with the incomplete prevention of future cerebral infarcts may decrease the enthusiasm for infarct recurrence prevention using regular blood transfusion therapy in children with preexisting SCI.
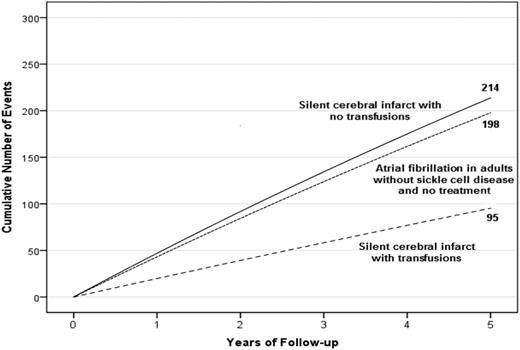
A hypothetical cohort of 1000 children with SCA and preexisting SCIs followed for 5 years. Events are defined as either SCIs or strokes for children with SCA. The figure depicts the infarct recurrence rate (overt and SCI) based on no therapy (4.5 events per 100 patient years) or regular blood transfusion (2.0 events per 100 patient years).7 To provide a frame of reference on the absolute number of children with infarct recurrence, we have included the expected number of strokes in 1000 adults without SCD, but with preexisting untreated atrial fibrillation.
A hypothetical cohort of 1000 children with SCA and preexisting SCIs followed for 5 years. Events are defined as either SCIs or strokes for children with SCA. The figure depicts the infarct recurrence rate (overt and SCI) based on no therapy (4.5 events per 100 patient years) or regular blood transfusion (2.0 events per 100 patient years).7 To provide a frame of reference on the absolute number of children with infarct recurrence, we have included the expected number of strokes in 1000 adults without SCD, but with preexisting untreated atrial fibrillation.
Primary stroke prevention in children in high-income countries is successful
The single greatest advance in preventing neurologic injury in children with SCA is initiating the use of TCD screening to identify a group of children at risk for future strokes: primary stroke prevention. The pivotal RCT demonstrated that children with elevated TCD measurements >200 cm per second receiving regular blood transfusion therapy (defined as transfusion every 3 to 6 weeks with a goal of keeping the maximum HbS level <30%), when compared with standard therapy (observation), will have an ∼92% relative risk reduction in the rate of overt strokes. The number with elevated TCD measurements receiving transfusion therapy to prevent 1 stroke was 7.4 When available, we prefer the use of erythrocytapheresis as the approach to regular transfusions because of the lower rate of iron accumulation.76
In tertiary care centers providing medical care for children with SCA, adherence to routine screening of children with SCA using TCD measurements, coupled with regular blood transfusion therapy, has dramatically reduced the rate of strokes. In 1 large tertiary care center the incidence rate of overt strokes declined a log-fold after the introduction of routine screening with TCD with blood transfusion therapy (0.67 before and 0.06 after strokes per 100 patient-years).77
Stroke With Transfusions Changing to Hydroxyurea (SWiTCH)6 was a secondary stroke-prevention trial in children with SCA. The primary objective of the trial was to complete a noninferiority randomized trial of hydroxyurea therapy and phlebotomy compared with blood transfusion therapy and chelation for 134 children with prior stroke who had already undergone at least 18 months of regular transfusion.6 The primary end point was a composite of recurrent stroke and liver iron concentration measured by MRI. There were no strokes in the 66 participants randomly allocated to transfusions and chelation (standard therapy), but 7 of the 67 participants (10%) randomly allocated to hydroxyurea therapy and phlebotomy (experimental therapy) had strokes. The trial was stopped for futility because there was no difference in liver iron concentration.6
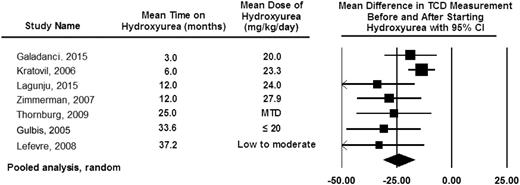
Based in part on consistent findings of studies demonstrating that hydroxyurea therapy lowers TCD measurements (Figure 7), the TCD With Transfusions Changing to Hydroxyurea (TWiTCH) Trial (NCT01425307)8 was funded. The main objective was a primary stroke-prevention trial for children with SCA who had received at least 12 months of blood transfusion therapy for TCD velocities above 200 cm per second. Children with SCA and prior elevated TCD velocities were randomly allocated to continue blood transfusion therapy and chelation (standard therapy [n = 61]) or start hydroxyurea therapy and phlebotomy (experimental therapy [n = 60]). Participants randomly allocated to the hydroxyurea and phlebotomy arm also simultaneously received blood transfusion therapy until the hydroxyurea maximum tolerated dose was reached (median ∼6 months overlap). Treatment was scheduled to last for 24 months after random allocation, at which point the primary outcome, TCD velocities, between the 2 arms was compared. After the first interim analysis, the trial was ended early because the noninferiority was demonstrated (margin of 15 cm per second).8
Pooled analysis of the 7 studies documenting TCD measurement before and after hydroxyurea therapy. The pooled analysis is based on the random-effect model demonstrating the average drop in TCD measurement after starting hydroxyurea therapy of 25 cm per second.80,81,95-99 The table also includes the observation that the decrease in TCD measurements can be seen as early as 3 months after starting hydroxyurea therapy with a sustained impact of hydroxyurea therapy on decreasing TCD measurements for at least 36 months. The black diamond represents the results of random-effect models. The edges of the diamonds represent the 95% CI of the meta-analyses for the random-effect models.26
Pooled analysis of the 7 studies documenting TCD measurement before and after hydroxyurea therapy. The pooled analysis is based on the random-effect model demonstrating the average drop in TCD measurement after starting hydroxyurea therapy of 25 cm per second.80,81,95-99 The table also includes the observation that the decrease in TCD measurements can be seen as early as 3 months after starting hydroxyurea therapy with a sustained impact of hydroxyurea therapy on decreasing TCD measurements for at least 36 months. The black diamond represents the results of random-effect models. The edges of the diamonds represent the 95% CI of the meta-analyses for the random-effect models.26
The management of children with both elevated TCD measurement and MRA-defined severe cerebral vasculopathy cannot be determined from the TWiTCH Trial. Regardless of vasculopathy status of children with elevated TCD measurements, the event rate of strokes is very low, 0.06 per 100 patient years in one tertiary care center,77 while receiving blood transfusion therapy. Furthermore, in ∼20% of the children with elevated TCD measurements, the TCD measurements will remain elevated several years after regular blood transfusion has begun with no evidence that this group is more likely to develop future strokes when compared with the group that drop their TCD measurement <200 cm per second after blood transfusion therapy.78
Given the success of hydroxyurea therapy for primary prevention of strokes and the relative low risk-to-benefit ratio when compared with hematopoietic stem cell transplant (HSCT), we would recommend continuing blood transfusion therapy or switching to hydroxyurea therapy, after at least one year of blood transfusion therapy over HSCT for the perceived high-risk group of children with MRA-defined vasculopathy and elevated TCD measurements. To date, there is no evidence that TCD measurement in individuals with SCA above 16 years of age is beneficial. In the only study to date, Valadi et al evaluated 110 adults with SCA and controls, and did not find any values that were elevated.79
Primary stroke prevention in low- and middle-income countries is just beginning
No definitive approach has been applied for primary stroke prevention in children with SCA living in Africa or India, where the majority of the children are born with SCA.9 Two strategies in Africa have been used, a standard care protocol using hydroxyurea therapy,80 or initially using a moderate fixed dose of hydroxyurea therapy of 20 mg/kg (Primary Stroke Prevention in Nigerian Children with Sickle Cell Disease [SPIN], NCT01801423).81 The feasibility trial included children with SCA between 5 and 12 years of age with elevated TCD velocities. The early results indicated that the families are willing to participate in a formal trial.81 Furthermore, in a short follow-up of participants and a comparison group (<18 months), there were initially no unwarranted toxicities when compared with a group of children with SCA who were screened, had TCD velocities <200 cm per second, and were followed prospectively. Perhaps most importantly, the early results suggest that children initially started on hydroxyurea therapy, instead of blood transfusion therapy, can have significant drops in their TCD measurements <200 cm per second 3 months after starting therapy.81
The essential question for primary stroke prevention in low- and middle-income countries is what dose of hydroxyurea therapy maximizes the benefit and minimizes the toxicity and laboratory surveillance costs. In a setting of high rates of life-threatening bacterial infections and malaria, treatment with hydroxyurea therapy, a myelosuppressive agent, may result in an increased rate of infections or other complications. Given the low median family income in urban northern Nigeria (<$700 per year),82 the out-of-pocket costs of a routine complete blood count (∼$5) for assessment of hydroxyurea therapy may be prohibitive. Thus, limiting the financial burden for complete blood count surveillance for hydroxyurea-related toxicity may decrease the financial barrier without potentially sacrificing safety. Based on the promising early results of the feasibility trial in Kano, Nigeria (SPIN, NCT01801423),81 the National Institute of Neurological Diseases and Stroke funded a RCT to determine the efficacy of 20 mg/kg per day vs 10 mg/kg per day of hydroxyurea therapy for primary stroke prevention in children with SCA living in Nigeria and Ghana (NCT02560935). For now, in low-income countries where TCD screening is initiated for primary stroke prevention and blood transfusion therapy is not routinely used, preliminary data suggest that a fixed dose of hydroxyurea therapy at 20 mg/kg per day is a reasonable starting point for treatment.81
Blood transfusion therapy for stroke prevention is palliative in high-income countries
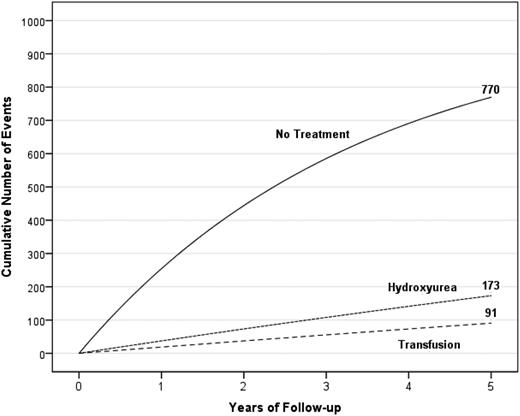
Standard treatment of secondary stroke prevention is blood transfusion therapy. However, even when blood transfusion therapy is initiated, 45% of the children with SCA will have infarct recurrence (both stroke and SCI) over a course of 5.5 years,66 providing evidence that alternative options must be considered for this high-risk population. Figure 8 depicts the unsatisfactory results of the treatment options for secondary infarct recurrence prevention in children with SCA and overt strokes.
A hypothetical cohort of 1000 children with SCA and strokes followed for 5 years receiving either no therapy, hydroxyurea therapy, or regular blood transfusion therapy. The figure depicts the number of children in the cohort with stroke recurrence in the no-treatment group, hydroxyurea therapy group, and regular blood transfusion therapy group with expected incidence rates of 29.1 (95% CI, 19.2-38.9), 3.8 (95% CI, 1.9-5.7), and 1.9 (95% CI, 1.0-2.9) events per 100 patient years, respectively.20
A hypothetical cohort of 1000 children with SCA and strokes followed for 5 years receiving either no therapy, hydroxyurea therapy, or regular blood transfusion therapy. The figure depicts the number of children in the cohort with stroke recurrence in the no-treatment group, hydroxyurea therapy group, and regular blood transfusion therapy group with expected incidence rates of 29.1 (95% CI, 19.2-38.9), 3.8 (95% CI, 1.9-5.7), and 1.9 (95% CI, 1.0-2.9) events per 100 patient years, respectively.20
Given the evidence that blood transfusion therapy is palliative for secondary stroke prevention,66 we believe that, after an initial stroke, alternative treatment options should be considered. Current evidence strongly suggests that HSCT decreases the rate of stroke recurrence83,84 ; however, most children and adults do not have a viable donor. A range of revascularization procedures has become an option for patients with internal carotid artery occlusion and moyamoya collaterals.85 Regrettably, no RCT has been introduced to rigorously assess standard care of regular blood transfusion therapy vs neurosurgery and regular blood transfusion therapy. Given the unknown risk-to-benefit ratio of alternative donor (haploidentical) or nonmyeloablative HSCT or revascularization procedures for secondary stroke prevention, we strongly recommend that single or preferably multi-institutional studies be registered with clinicaltrials.gov so we can collectively learn from these variations in practices with no clear superior strategy for secondary stroke prevention.
Strategies are just emerging for secondary prevention of strokes in low- and middle-income countries
Based on challenges of routine blood transfusion therapy in low- and middle-income countries, no definitive strategy has emerged for secondary stroke prevention. However, several investigators in these settings have elected to use hydroxyurea therapy as opposed to no therapy. Using pooled analysis comparing blood transfusion therapy to hydroxyurea therapy and no therapy, the expected stroke recurrence incidence rates were 1.9 (95% CI, 1.0-2.9), 3.8 (95% CI, 1.9-5.7), and 29.1 (95% CI, 19.2-38.9) events per 100 patient-years, respectively.20 A secondary stroke-prevention trial (National Clinical Trial [NCT] pending) is now funded in Nigeria to determine the efficacy for preventing stroke recurrence comparing 20 mg/kg per day vs 10 mg/kg per day of hydroxyurea therapy. The completion of the trial is not expected to occur for an additional 4 years. In the meantime, we believe that the available data suggest that treatment with hydroxyurea therapy for secondary prevention of strokes is a reasonable alternative when compared with no therapy at all.
Summary
As a direct result of completed RCTs (STOP, STOP II, SIT, SWiTCH, and TWiTCH)4-8 regarding children with SCA, a new standard of care has emerged for primary and secondary stroke prevention. Based on the preponderance of evidence, the use of hydroxyurea therapy clearly decreases TCD measurements, which is the main modifiable risk factor for overt strokes. Further bolstering the evidence of the impact of hydroxyurea therapy on decreasing TCD measurements, the TWiTCH trial has demonstrated the noninferiority of hydroxyurea therapy to blood for primary stroke prevention in children with SCA with high TCD velocities without vasculopathy on MRA who have already been transfused for a year. In low- and middle-income countries, where the majority of children with SCA are born and blood transfusion therapy is not routinely available, primary and secondary stroke-prevention RCTs are under way to determine the optimal hydroxyurea dose that maximizes benefits and limits toxicity, while potentially minimizing laboratory surveillance. Very few observational studies and no RCTs in adults with SCD have been undertaken for primary and secondary CNS complications (strokes, hemorrhage, and aneurysms). Although there have been significant strides in preventing CNS complications in the last 25 years, future research will need to focus on adults with SCD and individuals with SCD other than SCA, a group still at considerable risk for CNS morbidity.
The online version of this article contains a data supplement.
Authorship
Contribution: M.R.D. and F.J.K. contributed equally to writing this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Vanderbilt-Meharry Sickle Cell Center for Excellence, Vanderbilt University School of Medicine, 2200 Children’s Way, 11206 DOT, VCH, Nashville, TN 37232; e-mail: m.debaun@vanderbilt.edu.