In this issue of Blood, Walker et al estimate venous thromboembolism (VTE) rates associated with current breast cancer treatments in time windows before, during, and following treatment.1
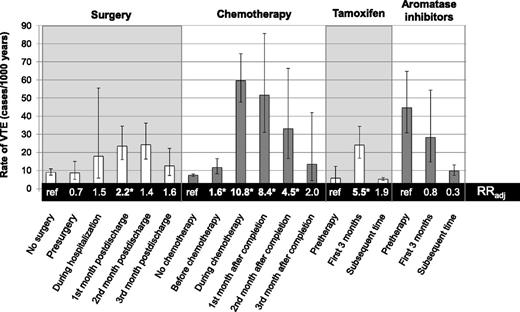
Absolute and adjusted relative rates (RRadj) of VTE associated with surgery, chemotherapy, and endocrine therapies over time. *RRs that are statistically significant at the level of P < .05. The figure is based on data from Table 2 in the article by Walker et al that begins on page 849.
Absolute and adjusted relative rates (RRadj) of VTE associated with surgery, chemotherapy, and endocrine therapies over time. *RRs that are statistically significant at the level of P < .05. The figure is based on data from Table 2 in the article by Walker et al that begins on page 849.
An increased burden of VTE among breast cancer patients is well established, and prior studies have identified patient-, disease-, and treatment-related characteristics associated with higher VTE risk, such as advanced stage and use of chemotherapy.2 In contrast to the approaches of prior observational studies and VTE prediction models for cancer patients,3,4 the current study provides data on the changing rates of VTE over the time course of treatment with chemotherapy, hormonal therapy, surgery, and clinical observation. By applying state-of-the-science statistical models that flexibly accommodate an individual patient’s changing therapies over time, Walker et al reveal dramatic changes in the rate of VTE over the course of a woman’s treatment of breast cancer.
In the present study, >13 000 women diagnosed with breast cancer between 1997 and 2006 were followed from the date of their cancer diagnosis for the incidence of VTE, as defined by a previously validated algorithm combining International Classification of Diseases–10 codes for VTE, anticoagulation treatment codes, and death data. VTE occurred in 611 (4.6%) women over the follow-up period, corresponding to an annualized rate of 0.84%/year, although this may underestimate the actual VTE rate (including more clinically silent VTE events only detectable by serial scans). Baseline risk factors of older age, overweight/obesity, and distant metastatic disease were associated with significantly increased VTE rates in multivariate-adjusted analyses. The accompanying figure illustrates how VTE rates change over time as patients are treated with chemotherapy and endocrine therapies or receive surgical intervention. Compared with patients not receiving surgery, surgical patients had a more than twofold increased rate of VTE in the 1-month interval following discharge from the surgical admission (hazard ratio [HR] = 2.2; 95% confidence interval [CI], 1.4-3.4), but not during other pre- or postoperative windows of time. The highest absolute rates of VTE were observed during chemotherapy and in the month following cessation of chemotherapy, at rates 10.8 and 8.4 times higher, respectively, than in women not treated with chemotherapy. Metastatic disease had a greater influence on VTE risk when women were not actively undergoing chemotherapy, although this finding is limited by a lack of complete staging information on one third of the cohort. Among women receiving tamoxifen therapy, the first 3 months of treatment were associated with a 5.5-fold increase in the rate of VTE compared with the pretreatment period, and risk remained elevated after 3 months (HR = 1.9; 95% CI, 0.9-4.3). In contrast, use of aromatase inhibitors was not associated with a significant change in the VTE rate over time.
Despite the morbidity and mortality associated with VTE in cancer patients, current clinical practice guidelines do not recommend routine thromboprophylaxis in outpatients with cancer.5,6 The relatively low overall rate of VTE among breast cancer patients, <1%/year in this study population, does not justify the notion of population-wide thromboprophylaxis given the risk of serious treatment-related adverse events such as major bleeding. A significant challenge is identifying the patients at moderate to high risk for VTE who are most likely to benefit from primary thromboprophylaxis treatment. Because the net benefit of therapies involving treatment-related harm depends on the likelihood of the outcome, a trial enrolling patients without a risk-based sampling strategy or subgroup analysis is unlikely to yield favorable summary results.7 The Evaluation of AVE5026 in the Prevention of Venous Thromboembolism in Cancer Patients Undergoing Chemotherapy trial demonstrated a large relative effect size (nearly threefold higher VTE risk in placebo vs semuloparin treated), but a small absolute risk reduction in VTE of only 2.2% (3.2% and 1.4% in control and treatment groups, respectively).8 Absolute risk reductions of this magnitude are not enough to counterbalance potential treatment related harms, assuming a 1% risk of intracranial hemorrhage. In contrast, the Charité Onkologie (CONKO)-004 trial selected patients with advanced pancreatic cancer, a malignancy associated with the highest VTE rates, and demonstrated a clinically significant absolute reduction in VTE risk from 15.1% to 6.4% in control vs treated patients.9
Although VTE is a relatively rare event, breast cancer is the most common cancer in women worldwide. Thus, the question of whether to use thromboprophylaxis to reduce breast cancer–related VTE has a substantial clinical impact. The findings by Walker et al suggest that the future of thromboprophylaxis in breast cancer patients should be time-limited treatment of those at highest risk. Selectively applying thromboprophylaxis to those patients at highest risk and, critically, only while those patients are at risk, may limit overtreatment of patients unlikely to benefit while avoiding the majority of adverse outcomes. Recognition of the time-sensitive nature of VTE risk associated with surgery and treatments could be leveraged to improve risk prediction algorithms to better identify candidates for thromboprophylaxis. Future studies are needed to evaluate how these results may be used to more precisely target patients with the best chance of benefiting from new and existing therapies. The current study provides valuable evidence that can both inform this future research and be used for counseling patients about VTE risk. Women with breast cancer undergoing surgery, chemotherapy, or hormonal therapy have a specific time interval during which they are at increased risk of VTE. Discussion of common VTE symptoms should therefore be included, alongside admonitions on neutropenic fevers and more common treatment side effects, as infrequent, although highly morbid, potential complications of therapy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.