Abstract
Immunotherapeutic strategies are emerging as promising therapeutic approaches in multiple myeloma (MM), with several monoclonal antibodies in advanced stages of clinical development. Of these agents, CD38-targeting antibodies have marked single agent activity in extensively pretreated MM, and preliminary results from studies with relapsed/refractory patients have shown enhanced therapeutic efficacy when daratumumab and isatuximab are combined with other agents. Furthermore, although elotuzumab (anti-SLAMF7) has no single agent activity in advanced MM, randomized trials in relapsed/refractory MM have demonstrated significantly improved progression-free survival when elotuzumab is added to lenalidomide-dexamethasone or bortezomib-dexamethasone. Importantly, there has been no significant additive toxicity when these monoclonal antibodies are combined with other anti-MM agents, other than infusion-related reactions specific to the therapeutic antibody. Prevention and management of infusion reactions is important to avoid drug discontinuation, which may in turn lead to reduced efficacy of anti-MM therapy. Therapeutic antibodies interfere with several laboratory tests. First, interference of therapeutic antibodies with immunofixation and serum protein electrophoresis assays may lead to underestimation of complete response. Strategies to mitigate interference, based on shifting the therapeutic antibody band, are in development. Furthermore, daratumumab, and probably also other CD38-targeting antibodies, interfere with blood compatibility testing and thereby complicate the safe release of blood products. Neutralization of the therapeutic CD38 antibody or CD38 denaturation on reagent red blood cells mitigates daratumumab interference with transfusion laboratory serologic tests. Finally, therapeutic antibodies may complicate flow cytometric evaluation of normal and neoplastic plasma cells, since the therapeutic antibody can affect the availability of the epitope for binding of commercially available diagnostic antibodies.
Introduction
In the last decade, survival of multiple myeloma (MM) patients has markedly improved.1 However, despite this progress, patients with disease refractory to both immunomodulatory drugs (IMiDs) and proteasome inhibitors have a median overall survival (OS) of only 9 months,2 highlighting the need for additional agents with novel mechanisms of action in this setting.3,4 Monoclonal antibodies (mAbs) are an important new class of agents, and results from recent clinical trials are very promising in MM.5-7
In this review, we will specifically focus on daratumumab (anti-CD38), which was approved in November 2015 by the US Food and Drug Administration (FDA) for treatment of MM patients who have received ≥3 prior therapies including a proteasome inhibitor and an IMiD or who are double-refractory to these drugs. We will also focus on other antibodies that have entered advanced stages of clinical testing including elotuzumab and the CD38-targeting antibodies isatuximab and MOR202. We will not discuss CD38-specific antibodies in preclinical development, such as Ab79 and Ab19 (Takeda). With the increased application of antibody-based therapies in MM, clinicians will now have to manage antibody-related adverse events, such as infusion-related reactions (IRRs). Furthermore, because many laboratory tests are based on specific antibody-antigen interactions, interference of therapeutic antibodies is increasingly being recognized. This includes interference of therapeutic antibodies with serum protein electrophoresis (SPEP), immunofixation electrophoresis (IFE), flow cytometric detection of minimal residual disease (MRD), and blood compatibility testing.
We will first review the mechanisms of action and clinical activity of these antibodies and then describe the management of clinically relevant adverse events, as well as the handling of interference of therapeutic antibodies with laboratory tests.
Mechanism of action of mAbs in MM
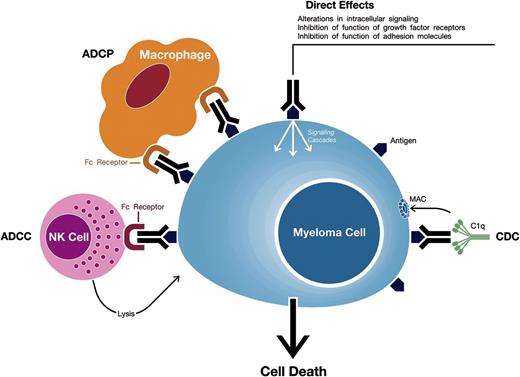
mAbs against target antigens expressed on MM cells can induce tumor cell killing via a variety of mechanisms including Fc-dependent effector mechanisms including complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP) (Figure 1).5,8,9 Importantly, they may also have direct effects via modulation of the activity of the targeted antigen.
Mechanisms of action of monoclonal antibodies targeting surface antigens on MM cells. Monoclonal antibodies against target antigens expressed on MM cells can induce tumor cell killing via Fc-dependent effector mechanisms including CDC, ADCC, and ADCP. The process of ADCC is achieved through activation of Fc receptors on myeloid and NK effector cells by tumor cell-attached immunoglobins. Subsequent cytotoxicity is mediated through ≥2 different mechanisms; one involving the release of perforin and granzymes from effector cells and the other involving death ligands FasL and tumor necrosis factor–related apoptosis-inducing ligand. In ADCP, phagocytosis of tumor cells is mediated by macrophages. CDC is dependent on the interaction of the antibody Fc domains with the classic complement-activating protein C1q leading to activation of downstream complement proteins, which results in the assembly of the membrane attack complex (MAC), that punches holes in the tumor cells. An additional result of this cascade is the production of chemotactic complement molecules C3a and C5a, which recruit and activate immune effector cells. There is also evidence that uptake of antibody-opsonized tumor cells and cellular fragments by antigen-presenting cells is associated with enhanced antigen presentation leading to tumor-specific T-cell responses. Monoclonal antibodies that target antigens on MM cells may also have direct effects via modulation of the activity of the targeted antigen.
Mechanisms of action of monoclonal antibodies targeting surface antigens on MM cells. Monoclonal antibodies against target antigens expressed on MM cells can induce tumor cell killing via Fc-dependent effector mechanisms including CDC, ADCC, and ADCP. The process of ADCC is achieved through activation of Fc receptors on myeloid and NK effector cells by tumor cell-attached immunoglobins. Subsequent cytotoxicity is mediated through ≥2 different mechanisms; one involving the release of perforin and granzymes from effector cells and the other involving death ligands FasL and tumor necrosis factor–related apoptosis-inducing ligand. In ADCP, phagocytosis of tumor cells is mediated by macrophages. CDC is dependent on the interaction of the antibody Fc domains with the classic complement-activating protein C1q leading to activation of downstream complement proteins, which results in the assembly of the membrane attack complex (MAC), that punches holes in the tumor cells. An additional result of this cascade is the production of chemotactic complement molecules C3a and C5a, which recruit and activate immune effector cells. There is also evidence that uptake of antibody-opsonized tumor cells and cellular fragments by antigen-presenting cells is associated with enhanced antigen presentation leading to tumor-specific T-cell responses. Monoclonal antibodies that target antigens on MM cells may also have direct effects via modulation of the activity of the targeted antigen.
Elotuzumab is a humanized immunoglobulin (Ig)G1-κ antibody targeting signaling lymphocytic activation molecule F7 (SLAMF7, also called CS1). SLAMF7 is expressed on MM cells, natural killer (NK) cells, and a subgroup of other immune cells. The mechanisms of action by which elotuzumab exerts its antitumor effects include ADCC10 and inhibition of the interactions between MM cells and stromal cells,11 but not CDC.11 In addition, elotuzumab directly enhances NK cell cytotoxicity via SLAMF7 ligation.12
Daratumumab (IgG1-κ; fully human), isatuximab (SAR650984; IgG1-κ; chimeric), and MOR202 (IgG1-λ; fully human) are CD38-targeting antibodies. CD38 is highly and ubiquitously expressed on MM cells and at low levels on normal lymphoid and myeloid cells.13 CD38 is a transmembrane glycoprotein with ectoenzymatic activity in the catabolism of extracellular nucleotides.14,15 Other functions include receptor-mediated adhesion by interacting with CD31 or hyalunoric acid, regulation of migration, and signaling events.15-18 The CD38-targeting antibodies kill MM cells via CDC, ADCC, ADCP, direct induction of apoptosis, and modulation of CD38 ectoenzyme function.19-28 Interestingly, although these antibodies target the same antigen and induce similar amounts of ADCC, marked differences in other effector functions such as CDC, ADCP, direct induction of apoptosis, and inhibition of CD38 ectoenzyme activity were observed when comparing different CD38 antibodies.19,20 It is currently unknown whether these differences in mechanisms of action translate into differential clinical activity.
Summary of the clinical results
Activity of mAbs as single agent
Elotuzumab is well tolerated, with adverse events primarily infusion related, but has no single agent activity in advanced MM.29 This contrasts with the CD38-targeting antibodies daratumumab and isatuximab, which have significant activity as monotherapy, although thus far the same degree of activity is not seen with MOR202 (Table 1).29-34
Selected studies with elotuzumab and CD38-targeting antibodies as single agent in relapsed/refractory MM
| Study . | Type of study . | Regimen . | Schedule . | N . | Prior treatment . | Response . | TTE . | Key toxicities . |
|---|---|---|---|---|---|---|---|---|
| Zonder et al29 | Phase 1 | Elotuzumab | MTD not reached; highest dose-level: elotuzumab 20 mg/kg iv; administered once every 2 weeks for 8 weeks; patients without PD or relapse at week 8 had the option to receive a second 8-week treatment course | 34 | Median: 4.5 Range: 2-10 Thal: 79.4% Len: 82.4% Bort: 82.4% | ≥PR: 0% SD: 26.5% | Not reported | IRR before institution of infusion prophylaxis: 52% |
| Lokhorst et al30 (GEN501) | Phase 1/2 | Daratumumab | MTD not reached; highest dose level: daratumumab 24 mg/kg iv; in part 2, daratumumab 8 or 16 mg/kg iv was administered once-weekly for 8 times (schedules D and E: 3-week wash out period after infusion 1), and then twice-monthly for 14-16 weeks, followed by monthly infusions until PD/unacceptable toxicity | Phase 2 with daratumumab 16 mg/kg: 42 | Phase 2 with daratumumab 16 mg/kg; Median: 4 Range: 2-12 Thal-refractory: 29% Len-refractory: 74% Bort-refractory: 71% Carf-refractory: 17% Pom-refractory: 36% | Phase 2 with daratumumab 16 mg/kg; ≥PR: 36% VGPR: 5% CR: 5% | Phase 2 with daratumumab 16 mg/kg; Median PFS: 5.6 months 1-year OS: 77% | Phase 2 with daratumumab 16 mg/kg; IRR: 74%; mostly grade 1/2 (grade 3 in 2%) |
| Lonial et al31 (Sirius) | Phase 2 | Daratumumab | Initially daratumumab 8 or 16 mg/kg; 16 mg/kg was established as the recommended dose for further study. Daratumumab 16 mg/kg was given weekly for 8 weeks, every 2 weeks for 16 weeks, followed by monthly infusions | Daratumumab 16 mg/kg: 106 | Daratumumab 16 mg/kg; Median: 5 Range: 2-14 Thal: 44% Len-refractory: 88% Bort-refractory: 90% Carf-refractory: 48% Pom-refractory: 63% | Daratumumab 16 mg/kg; ≥PR: 29% VGPR: 9% CR: 3% | Daratumumab 16 mg/kg; Median PFS: 3.7 months 1-year OS: 65% | Daratumumab 16 mg/kg; IRR: 43%; mostly grade 1/2 (grade 3 in 5%) |
| Martin et al32,33 | Phase 1 | Isatuximab | MTD not reached; highest dose-level: isatuximab 20 mg/kg iv; isatuximab was administered every 2 weeks or weekly | Total patients with CD38+ hematological malignancies: 39MM patients: 35 | MM patients; Median: 6 Range: 2-14 IMiD and PI: 97% Carf and/or pom: 57% | MM patients treated with isatuximab ≥ 10 mg/kg (n=18); ≥PR: 33% CR: 11% | MM patients; Not reported | MM patients treated with isatuximab ≥10 mg/kg; IRR: 43% (mostly grade 1/2) |
| Raab et al34 | Phase 1/2a | MOR202 | MTD not reached; highest dose-level: MOR202 16 mg/kg iv; MOR202 was administered every 2 weeks or weekly | 45 | Median: 4 Range: not reported Thal: 36% Len: 98% Bort: 100% Carf: 7% Pom: 16% | MOR202 ≥ 1.5 mg/kg (n = 24); ≥PR: 8.3% VGPR: 4.2% | Not reported | IRR: 40% in patients without dexamethasone as premedication, mostly grade 1/2 (grade 3 in 3%) IRR: 10% in patients with dexamethasone as premedication |
| Study . | Type of study . | Regimen . | Schedule . | N . | Prior treatment . | Response . | TTE . | Key toxicities . |
|---|---|---|---|---|---|---|---|---|
| Zonder et al29 | Phase 1 | Elotuzumab | MTD not reached; highest dose-level: elotuzumab 20 mg/kg iv; administered once every 2 weeks for 8 weeks; patients without PD or relapse at week 8 had the option to receive a second 8-week treatment course | 34 | Median: 4.5 Range: 2-10 Thal: 79.4% Len: 82.4% Bort: 82.4% | ≥PR: 0% SD: 26.5% | Not reported | IRR before institution of infusion prophylaxis: 52% |
| Lokhorst et al30 (GEN501) | Phase 1/2 | Daratumumab | MTD not reached; highest dose level: daratumumab 24 mg/kg iv; in part 2, daratumumab 8 or 16 mg/kg iv was administered once-weekly for 8 times (schedules D and E: 3-week wash out period after infusion 1), and then twice-monthly for 14-16 weeks, followed by monthly infusions until PD/unacceptable toxicity | Phase 2 with daratumumab 16 mg/kg: 42 | Phase 2 with daratumumab 16 mg/kg; Median: 4 Range: 2-12 Thal-refractory: 29% Len-refractory: 74% Bort-refractory: 71% Carf-refractory: 17% Pom-refractory: 36% | Phase 2 with daratumumab 16 mg/kg; ≥PR: 36% VGPR: 5% CR: 5% | Phase 2 with daratumumab 16 mg/kg; Median PFS: 5.6 months 1-year OS: 77% | Phase 2 with daratumumab 16 mg/kg; IRR: 74%; mostly grade 1/2 (grade 3 in 2%) |
| Lonial et al31 (Sirius) | Phase 2 | Daratumumab | Initially daratumumab 8 or 16 mg/kg; 16 mg/kg was established as the recommended dose for further study. Daratumumab 16 mg/kg was given weekly for 8 weeks, every 2 weeks for 16 weeks, followed by monthly infusions | Daratumumab 16 mg/kg: 106 | Daratumumab 16 mg/kg; Median: 5 Range: 2-14 Thal: 44% Len-refractory: 88% Bort-refractory: 90% Carf-refractory: 48% Pom-refractory: 63% | Daratumumab 16 mg/kg; ≥PR: 29% VGPR: 9% CR: 3% | Daratumumab 16 mg/kg; Median PFS: 3.7 months 1-year OS: 65% | Daratumumab 16 mg/kg; IRR: 43%; mostly grade 1/2 (grade 3 in 5%) |
| Martin et al32,33 | Phase 1 | Isatuximab | MTD not reached; highest dose-level: isatuximab 20 mg/kg iv; isatuximab was administered every 2 weeks or weekly | Total patients with CD38+ hematological malignancies: 39MM patients: 35 | MM patients; Median: 6 Range: 2-14 IMiD and PI: 97% Carf and/or pom: 57% | MM patients treated with isatuximab ≥ 10 mg/kg (n=18); ≥PR: 33% CR: 11% | MM patients; Not reported | MM patients treated with isatuximab ≥10 mg/kg; IRR: 43% (mostly grade 1/2) |
| Raab et al34 | Phase 1/2a | MOR202 | MTD not reached; highest dose-level: MOR202 16 mg/kg iv; MOR202 was administered every 2 weeks or weekly | 45 | Median: 4 Range: not reported Thal: 36% Len: 98% Bort: 100% Carf: 7% Pom: 16% | MOR202 ≥ 1.5 mg/kg (n = 24); ≥PR: 8.3% VGPR: 4.2% | Not reported | IRR: 40% in patients without dexamethasone as premedication, mostly grade 1/2 (grade 3 in 3%) IRR: 10% in patients with dexamethasone as premedication |
bort, bortezomib; carf, carfilzomib; CR, complete response; dex, dexamethasone; IRR, infusion-related reaction; len, lenalidomide; PD, progressive disease; PFS, progression-free survival; PI, proteasome inhibitor; pom, pomalidomide; PR, partial response; thal, thalidomide; TTE, time to events; VGPR, very good partial response.
In the first-in-human study with daratumumab (GEN501), the maximum tolerated dose (MTD) was not reached with dose levels up to 24 mg/kg. In the phase 2 part of this study, at least a PR was achieved in 36% of extensively pretreated patients (median, 4 prior therapies) who received daratumumab at a dose of 16 mg/kg.30 The median PFS was 5.6 months and 1-year OS was 77%. The Sirius study (median, 5 prior therapies) confirmed these results with at least a PR in 29% and stringent CR in 3% of patients treated with 16 mg/kg daratumumab. Importantly, at least a PR was achieved in 21% of patients who were refractory to bortezomib, lenalidomide, pomalidomide, and carfilzomib.31 This suggests that mechanisms of resistance toward prior therapies do not affect the susceptibility of MM cells to daratumumab. In the Sirius study, the median PFS was 3.7 months with a 1-year OS of 65%.31 Daratumumab was well tolerated, with infusion reactions as the most frequent adverse events.30,31 Based on these data, the FDA granted, in November 2015, accelerated approval for daratumumab to treat MM patients who have received ≥3 prior treatments including a proteasome inhibitor and an IMiD or who are double-refractory to a proteasome inhibitor and an IMiD.
A phase 1 dose-escalation study with diverse CD38-positive hematologic malignancies, including 34 relapsed/refractory MM patients, evaluated the safety and efficacy of isatuximab. The MTD was not reached with dosing up to 20 mg/kg. In the subgroup of MM patients (median of 6 prior lines of therapy, n = 18), isatuximab at a dose of ≥10 mg/kg was well tolerated and induced at least a PR in 33% including CR in 11%.32,33
Combination therapy in relapsed/refractory MM
Because mAbs have a favorable toxicity profile and distinct mechanisms of action, when compared with established anti-MM agents, mAbs are attractive partners in combination regimens.5
Combinations with IMiDs
Preclinical evidence shows that lenalidomide enhances anti-MM activity of elotuzumab and CD38-targeting mAbs via activating the effector cells of ADCC,11,22,26,37-41 which formed the rationale for the clinical evaluation of these combinations (Table 2).42-47
Selected studies with elotuzumab and CD38-targeting antibodies in combination with other agents in relapsed/refractory MM
| Study . | Type of study . | Regimen . | Schedule . | N . | Prior treatment . | Response . | TTE . | Key toxicities . |
|---|---|---|---|---|---|---|---|---|
| Lonial et al42 | Phase 3 | Elotuzumab plus len-dex Len-dex | Elotuzumab: 10 mg/kg iv on days 1, 8, 15, and 22 during first 2 cycles and then on days 1 and 15 starting with third cycle Len: 25 mg by mouth on days 1-21 of 28-day cycle Dex: once weekly, on elotuzumab days 8 mg iv and 28 mg by mouth, on other days 40 mg by mouth Len: 25 mg on days 1-21 of 28-day cycle Dex: 40 mg once weekly | 646 | Median: 2 Range: 1-4 Thal: 48% Len (not refractory): 6% Bort: 70% | ≥PR: 79% VGPR: 28% CR: 4% ≥PR: 66% VGPR: 21% CR: 7% | Median PFS: 19.4 months Median PFS: 14.9 months | Grade 3/4 lymphopenia: 77% Herpes zoster: 4.1 per 100 patient-years IRR: 10% (mostly grade 1/2) Grade 3/4 lymphopenia: 49% Herpes zoster: 2.2 per 100 patient-years |
| Jakubowiak et al43 | Randomized phase 2 | Elotuzumab plus bort-dex Bort-dex | Elotuzumab: 10 mg/kg iv on days 1, 8, and 15 during cycles 1-2; on days 1 and 11 during cycles 3-8; cycle 9+: on days 1 and 15 Bort: 1.3 mg/m2 iv or sc on days 1, 4, 8, and 11 of 21-day cycle, during cycles 1-8; cycle 9+: on days 1, 8, and 15 of 28-d cycle Dex: 20 mg by mouth (8 mg iv and 8 mg by mouth on elotuzumab dosing days) Bort: 1.3 mg/m2 iv or sc on days 1, 4, 8, and 11 of 21-day cycle, during cycles 1-8; cycle 9+: on days 1, 8, and 15 of 28-day cycle Dex: 20 mg by mouth | 152 | Median: 1 Range: 1-3 Prior IMiD: 74% Prior PI (not refractory): 52% | ≥PR: 65% VGPR: 30% CR: 4% ≥PR: 63% VGPR: 23% CR: 4% | Median PFS: 9.7 months 1-year OS: 85% Median PFS: 6.9 months 1-year OS: 74% | IRR in elotuzumab group: 7% (all grade 1/2); no additional events in elotuzumab group compared with control group |
| Plesner et al44,45 (GEN503) | Phase 1/2 | Daratumumab plus len-dex | MTD not reached; highest dose-level: Daratumumab 16 mg/kg iv weekly during the first 2 cycles, every other week during cycles 3-6, and monthly in cycle 7 and beyond until PD or unacceptable toxicity Len: 25 mg by mouth on days 1-21 of 28-day cycle Dex: 40 mg weekly | Phase 2 with daratumumab 16 mg/kg: 32 | Phase 2 with daratumumab 16 mg/kg; Median: 2 Range: 1-3 Thal: not reported Len (not refractory): 34% Bort: not reported | Phase 2 with daratumumab 16 mg/kg; ≥PR: 88% VGPR: 28% CR: 25% | Phase 2 with daratumumab 16 mg/kg Not reported | Phase 2 with daratumumab 16 mg/kg; IRR: 56%, mostly grade 1/2 (grade 3 in 6%) |
| Chari et al46 | Phase 1b | Daratumumab plus pom-dex | Daratumumab 16 mg/kg iv weekly during the first 2 cycles, every other week during cycles 3-6, and monthly in cycle 7 and beyond until PD or unacceptable toxicity Pom: 4 mg by mouth on days 1-21 of 28-day cycle Dex: 40 mg weekly (20 mg for patients >75 years of age) | 77 | Median: 3.5 Range: 2-10 Thal-refractory: not reported: Len-refractory: 88% Bort-refractory: 65% Carf-refractory: 30% PI and IMID-refractory: 65% | 53 patients with > 1 post-baseline assessment; ≥ PR: 59% VGPR: 23% CR: 8% Double-refractory patients (n=40); ≥ PR: 58% | Not reported | IRR: 61%; little additional toxicity when daratumumab was added to pom-dex |
| Martin et al47 | Phase 1b | Isatuximab plus len-dex | MTD not reached; highest dose-level: Isatuximab 10 mg/kg iv on days 1 and 15 of each 28-day cycle Len: 25 mg by mouth on days 1-21 of 28-d cycle Dex: 40 mg weekly | Isatuximab 10 mg/kg: 24Whole group: 31 | Isatuximab 10 mg/kg; Median: 4 Range: 1-9 Thal-refractory: NA Len-refractory: ∼80% Bort-refractory: ∼60% Carf-refractory: ∼50% Pom-refractory: ∼30% | Isatuximab 10 mg/kg; ≥PR: 63% VGPR: 29% CR: 8% | Whole group Median PFS: 6.2 months | Whole group; IRR: 39% (grade 3 in 6%) |
| Study . | Type of study . | Regimen . | Schedule . | N . | Prior treatment . | Response . | TTE . | Key toxicities . |
|---|---|---|---|---|---|---|---|---|
| Lonial et al42 | Phase 3 | Elotuzumab plus len-dex Len-dex | Elotuzumab: 10 mg/kg iv on days 1, 8, 15, and 22 during first 2 cycles and then on days 1 and 15 starting with third cycle Len: 25 mg by mouth on days 1-21 of 28-day cycle Dex: once weekly, on elotuzumab days 8 mg iv and 28 mg by mouth, on other days 40 mg by mouth Len: 25 mg on days 1-21 of 28-day cycle Dex: 40 mg once weekly | 646 | Median: 2 Range: 1-4 Thal: 48% Len (not refractory): 6% Bort: 70% | ≥PR: 79% VGPR: 28% CR: 4% ≥PR: 66% VGPR: 21% CR: 7% | Median PFS: 19.4 months Median PFS: 14.9 months | Grade 3/4 lymphopenia: 77% Herpes zoster: 4.1 per 100 patient-years IRR: 10% (mostly grade 1/2) Grade 3/4 lymphopenia: 49% Herpes zoster: 2.2 per 100 patient-years |
| Jakubowiak et al43 | Randomized phase 2 | Elotuzumab plus bort-dex Bort-dex | Elotuzumab: 10 mg/kg iv on days 1, 8, and 15 during cycles 1-2; on days 1 and 11 during cycles 3-8; cycle 9+: on days 1 and 15 Bort: 1.3 mg/m2 iv or sc on days 1, 4, 8, and 11 of 21-day cycle, during cycles 1-8; cycle 9+: on days 1, 8, and 15 of 28-d cycle Dex: 20 mg by mouth (8 mg iv and 8 mg by mouth on elotuzumab dosing days) Bort: 1.3 mg/m2 iv or sc on days 1, 4, 8, and 11 of 21-day cycle, during cycles 1-8; cycle 9+: on days 1, 8, and 15 of 28-day cycle Dex: 20 mg by mouth | 152 | Median: 1 Range: 1-3 Prior IMiD: 74% Prior PI (not refractory): 52% | ≥PR: 65% VGPR: 30% CR: 4% ≥PR: 63% VGPR: 23% CR: 4% | Median PFS: 9.7 months 1-year OS: 85% Median PFS: 6.9 months 1-year OS: 74% | IRR in elotuzumab group: 7% (all grade 1/2); no additional events in elotuzumab group compared with control group |
| Plesner et al44,45 (GEN503) | Phase 1/2 | Daratumumab plus len-dex | MTD not reached; highest dose-level: Daratumumab 16 mg/kg iv weekly during the first 2 cycles, every other week during cycles 3-6, and monthly in cycle 7 and beyond until PD or unacceptable toxicity Len: 25 mg by mouth on days 1-21 of 28-day cycle Dex: 40 mg weekly | Phase 2 with daratumumab 16 mg/kg: 32 | Phase 2 with daratumumab 16 mg/kg; Median: 2 Range: 1-3 Thal: not reported Len (not refractory): 34% Bort: not reported | Phase 2 with daratumumab 16 mg/kg; ≥PR: 88% VGPR: 28% CR: 25% | Phase 2 with daratumumab 16 mg/kg Not reported | Phase 2 with daratumumab 16 mg/kg; IRR: 56%, mostly grade 1/2 (grade 3 in 6%) |
| Chari et al46 | Phase 1b | Daratumumab plus pom-dex | Daratumumab 16 mg/kg iv weekly during the first 2 cycles, every other week during cycles 3-6, and monthly in cycle 7 and beyond until PD or unacceptable toxicity Pom: 4 mg by mouth on days 1-21 of 28-day cycle Dex: 40 mg weekly (20 mg for patients >75 years of age) | 77 | Median: 3.5 Range: 2-10 Thal-refractory: not reported: Len-refractory: 88% Bort-refractory: 65% Carf-refractory: 30% PI and IMID-refractory: 65% | 53 patients with > 1 post-baseline assessment; ≥ PR: 59% VGPR: 23% CR: 8% Double-refractory patients (n=40); ≥ PR: 58% | Not reported | IRR: 61%; little additional toxicity when daratumumab was added to pom-dex |
| Martin et al47 | Phase 1b | Isatuximab plus len-dex | MTD not reached; highest dose-level: Isatuximab 10 mg/kg iv on days 1 and 15 of each 28-day cycle Len: 25 mg by mouth on days 1-21 of 28-d cycle Dex: 40 mg weekly | Isatuximab 10 mg/kg: 24Whole group: 31 | Isatuximab 10 mg/kg; Median: 4 Range: 1-9 Thal-refractory: NA Len-refractory: ∼80% Bort-refractory: ∼60% Carf-refractory: ∼50% Pom-refractory: ∼30% | Isatuximab 10 mg/kg; ≥PR: 63% VGPR: 29% CR: 8% | Whole group Median PFS: 6.2 months | Whole group; IRR: 39% (grade 3 in 6%) |
Elotuzumab plus lenalidomide-dexamethasone was well tolerated with encouraging response rate in a phase 1 study and phase 2 study.48-50 This led to the initiation of a randomized phase 3 trial (ELOQUENT-2) in relapsed/refractory MM (1-3 prior therapies), which showed that elotuzumab (10 mg/kg) combined with lenalidomide-dexamethasone improved median PFS compared with lenalidomide-dexamethasone alone (19.4 vs 14.9 months).42 This PFS benefit was consistent across key subgroups, including patients ≥65 years of age, with del(17p), or with a creatinine clearance of <60 mL/min.42 Also, the overall response rate was higher in the elotuzumab group compared with the control group (79% vs 66%).42 After adjustment for drug exposure, rates of infection were equal in the 2 treatment arms. However, there was an increased rate of herpes zoster infection in the elotuzumab group (incidence per 100 patient-years, 4.1 vs 2.2). The underlying cause is currently unknown, but the increased herpes zoster frequency may be related to the more frequent grade 3/4 lymphopenia in elotuzumab-treated patients (77% vs 49%).42 Change from baseline for diverse lymphocyte subgroups has not been reported. Rates for grade 3/4 anemia, thrombocytopenia, neutropenia, cardiac disorders, and renal disorders were similar between both groups.42 Based on these data, the FDA approved in November 2015 elotuzumab in combination with lenalidomide and dexamethasone for the treatment of patients with MM who have received 1-3 prior therapies.
Preliminary results from an ongoing phase 1/2 study of daratumumab with lenalidomide-dexamethasone in relapsed/refractory MM showed a high response rate that improved over time (phase 2 part with 16 mg/kg daratumumab, ≥PR: 88%; CR: 25%).44 Based on these results, a phase 3 randomized study, whose enrollment is now completed, compares lenalidomide-dexamethasone with or without daratumumab in the relapsed/refractory setting (Pollux trial). Similarly, preliminary data from a phase 1 trial showed that isatuximab in combination with lenalidomide-dexamethasone is effective and has a favorable toxicity profile.47 Interestingly, response was also achieved in lenalidomide-refractory patients (≥PR: 48%), which suggests that the immune system of these patients could still respond to the immunomodulatory effects of lenalidomide.47 MOR202 will also be evaluated in combination with lenalidomide-dexamethasone.35,36
Because pomalidomide has potent immune stimulating activity,51 it is another interesting partner drug for therapeutic antibodies.28 A phase 1b study is currently enrolling patients to evaluate daratumumab combined with pomalidomide and dexamethasone in relapsed/refractory MM. Preliminary data show high efficacy with at least a PR in 58% of double-refractory patients and little added toxicity.46,52 The combination of pomalidomide-dexamethasone with isatuximab or MOR202 will also be evaluated in relapsed/refractory MM.36
Combinations with bortezomib
Preclinical studies showing that the efficacy of elotuzumab and CD38-targeting antibodies was enhanced by bortezomib formed the rationale for the clinical evaluation of the combination of bortezomib and a therapeutic antibody (Table 2).11,37,38,53,54
A phase 1 study in relapsed/refractory MM showed encouraging activity of elotuzumab combined with bortezomib with at least a PR in 48% of patients and median PFS of 9.5 months.55 This study was followed by a randomized phase 2 trial that compared elotuzumab plus bortezomib-dexamethasone vs bortezomib-dexamethasone alone in MM patients with 1 to 3 prior therapies.43 The median PFS was significantly longer in the elotuzumab group compared with the group of patients treated with bortezomib-dexamethasone alone (median PFS, 9.7 vs 6.9 months). Moreover, there was limited added toxicity when elotuzumab was combined with bortezomib-dexamethasone compared with bortezomib-dexamethasone alone.43
Several studies are ongoing that evaluate the combination of a CD38-targeting antibody and bortezomib. This includes the phase 3 Castor study, which is enrolling relapsed/refractory MM patients to evaluate bortezomib-dexamethasone with or without daratumumab. Other ongoing studies in the relapsed/refractory setting will assess the addition of isatuximab or daratumumab to carfilzomib.
Newly diagnosed MM
Several trials are now evaluating the incorporation of elotuzumab, daratumumab, and isatuximab in backbone regimens for the treatment of both transplant eligible and elderly patients with newly diagnosed MM (Table 3). Furthermore, these antibodies alone or in combination are also evaluated in high-risk smoldering MM (Table 4).
Selected ongoing studies with elotuzumab- and CD38-targeting antibody-containing regimens in newly diagnosed MM
| Study . | Phase . | Setting . | Treatment . |
|---|---|---|---|
| NCT01335399 (ELOQUENT-1) | 3 | Nontransplant eligible | Lenaldiomide-dexamethasone vs lenalidomide-dexamethasone with elotuzumab |
| NCT02420860 | 2 | Transplant eligible | Elotuzumab with lenalidomide as maintenance after auto-SCT |
| NCT02495922 | 3 | Transplant eligible | Four study arms A1: RVD induction–auto-SCT–RVD consolidation–lenalidomide and dexamethasone maintenance A2: RVD induction–auto-SCT–RVD with elotuzumab consolidation–lenalidomide and dexamethasone with elotuzumab maintenance B1: RVD with elotuzumab induction–auto-SCT–RVD consolidation–lenalidomide and dexamethasone maintenance B2: RVD with elotuzumab induction–auto-SCT–RVD with elotuzumab consolidation–lenalidomide and dexamethasone with elotuzumab maintenance |
| NCT02375555 | 2a | Transplant eligible | Elotuzumab plus bortezomib, lenalidomide and dexamethasone (patients have option to undergo auto-SCT) |
| NCT01668719 | 1/2 | High-risk MM and age ≥18 years | Phase 1: Elotuzumab plus bortezomib, lenalidomide, and dexamethasone Phase 2: bortezomib-lenalidomide-dexamethasone vs bortezomib-lenalidomide-dexamethasone with elotuzumab |
| NCT01998971 | 1b | Irrespective of transplant eligibility for VD and VTD, and transplant ineligible for VMP | Daratumumab combined with VD, VTD, or VMP |
| NCT02252172 (Maia) | 3 | Nontransplant eligible | Lenalidomide-dexamethasone vs lenalidomide-dexamethasone plus daratumumab |
| NCT02195479 (Alcyone) | 3 | Nontransplant eligible | VMP vs VMP with daratumumab |
| NCT02541383 (Cassiopeia; IFM 2015-01; HOVON 131) | 3 | Transplant eligible | Randomization 1: VTD induction therapy–high-dose melphalan plus autologous stem cell rescue–VTD consolidation vs VTD with daratumumab induction therapy–high-dose melphalan plus autologous stem cell rescue–VTD with daratumumab consolidation Randomization 2: daratumumab as single agent in maintenance vs observation only |
| NCT02513186 | 1 | Nontransplant eligible | Isatuximab in combination with CyBorD |
| Study . | Phase . | Setting . | Treatment . |
|---|---|---|---|
| NCT01335399 (ELOQUENT-1) | 3 | Nontransplant eligible | Lenaldiomide-dexamethasone vs lenalidomide-dexamethasone with elotuzumab |
| NCT02420860 | 2 | Transplant eligible | Elotuzumab with lenalidomide as maintenance after auto-SCT |
| NCT02495922 | 3 | Transplant eligible | Four study arms A1: RVD induction–auto-SCT–RVD consolidation–lenalidomide and dexamethasone maintenance A2: RVD induction–auto-SCT–RVD with elotuzumab consolidation–lenalidomide and dexamethasone with elotuzumab maintenance B1: RVD with elotuzumab induction–auto-SCT–RVD consolidation–lenalidomide and dexamethasone maintenance B2: RVD with elotuzumab induction–auto-SCT–RVD with elotuzumab consolidation–lenalidomide and dexamethasone with elotuzumab maintenance |
| NCT02375555 | 2a | Transplant eligible | Elotuzumab plus bortezomib, lenalidomide and dexamethasone (patients have option to undergo auto-SCT) |
| NCT01668719 | 1/2 | High-risk MM and age ≥18 years | Phase 1: Elotuzumab plus bortezomib, lenalidomide, and dexamethasone Phase 2: bortezomib-lenalidomide-dexamethasone vs bortezomib-lenalidomide-dexamethasone with elotuzumab |
| NCT01998971 | 1b | Irrespective of transplant eligibility for VD and VTD, and transplant ineligible for VMP | Daratumumab combined with VD, VTD, or VMP |
| NCT02252172 (Maia) | 3 | Nontransplant eligible | Lenalidomide-dexamethasone vs lenalidomide-dexamethasone plus daratumumab |
| NCT02195479 (Alcyone) | 3 | Nontransplant eligible | VMP vs VMP with daratumumab |
| NCT02541383 (Cassiopeia; IFM 2015-01; HOVON 131) | 3 | Transplant eligible | Randomization 1: VTD induction therapy–high-dose melphalan plus autologous stem cell rescue–VTD consolidation vs VTD with daratumumab induction therapy–high-dose melphalan plus autologous stem cell rescue–VTD with daratumumab consolidation Randomization 2: daratumumab as single agent in maintenance vs observation only |
| NCT02513186 | 1 | Nontransplant eligible | Isatuximab in combination with CyBorD |
auto-SCT, autologous stem cell transplantation; RVD, lenalidomide, bortezomib, and dexamethasone; VD, bortezomib-dexamethasone; VMP, bortezomib, melphalan and prednisone; CyBorD, cyclophosphamide, bortezomib, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone.
Selected ongoing studies with elotuzumab- and daratumumab-containing regimens in previously untreated high-risk smoldering MM
| Study . | Phase . | Treatment . |
|---|---|---|
| NCT02316106 (Centaurus) | 2 | Daratumumab as single agent |
| NCT02279394 | 2 | Elotuzumab and lenalidomide ± dexamethasone |
| NCT01441973 | 2 | Elotuzumab as single agent |
| Study . | Phase . | Treatment . |
|---|---|---|
| NCT02316106 (Centaurus) | 2 | Daratumumab as single agent |
| NCT02279394 | 2 | Elotuzumab and lenalidomide ± dexamethasone |
| NCT01441973 | 2 | Elotuzumab as single agent |
Resistance to antibody-based therapy
Therapeutic antibodies have impressive activity in MM, but there is marked heterogeneity in response. Furthermore, the majority of responding patients will eventually develop resistance to mAbs.5,56 Improved understanding of mechanisms that contribute to innate or acquired resistance, may result in the rational design of new antibody-based combinations with higher anti-MM activity.
Preclinical experiments showed that daratumumab-mediated ADCC and CDC are associated with CD38 expression on the tumor cells.24 This presumably explains why patients who respond to daratumumab monotherapy have higher CD38 expression on their MM cells compared with nonresponders.57 Interestingly, all-trans retinoic acid improves daratumumab-mediated ADCC and CDC by upregulation of CD38 expression,24 suggesting that increasing target expression levels with all-trans retinoic acid may be a novel strategy to enhance the effectiveness of daratumumab. To what extent target expression is associated with response to other mAbs is currently under investigation. Downregulation of the target antigen during antibody therapy, as has been demonstrated for CD38, may contribute to development of acquired resistance.57
The presence of soluble forms of CD3858 and SLAMF711 may also affect outcome of antibody therapy. Similarly, development of anti-idiotype antibodies that neutralize the therapeutic antibody may be associated with reduced biological activity. Antidrug antibodies developed in 39% of patients treated with elotuzumab as single agent.29 In the lower-dose groups, the development of these antibodies was more common, and their impact on elotuzumab serum concentrations was more pronounced.29 In the ELOQUENT-2 study, 15% of patients tested positive for antidrug antibodies on ≥1 occasion.42 Until now, anti-daratumumab (Dr K. Sasser, Janssen Pharmaceuticals, written communication, November 2, 2015) and anti-isatuximab antibodies were not detected.32 Furthermore, only 1 of 50 MOR202-treated patients developed a transient anti-MOR202 antibody response.34
Subgroup analysis suggests that mAbs are also active in patients with high-risk cytogenetics. Daratumumab induced at least a PR in 20% of patients with high-risk cytogenetics.31 Furthermore, addition of elotuzumab to lenalidomide-dexamethasone also improved the outcome of patients with del(17p), t(4;14), and aml(1q).42 However, these subgroups involve small numbers of patients, and further analysis is needed to assess the impact of high-risk cytogenetics on outcome with antibody-based therapies.
Other factors that may influence clinical activity of therapeutic antibodies in MM include expression levels of complement-inhibitory proteins on MM cells,57 frequency and activity of effector cells,24 and immunogenetic factors contributing to effector cell function such as Fcγ receptor polymorphisms,5 as well as KIR and HLA genotypes.59
Management of adverse events
General considerations
Although CD38 and SLAMF7 expression is not restricted to MM cells, these mAbs have a very favorable toxicity profile, with IRRs as the most common side effect. Importantly, tolerability of elotuzumab and daratumumab in elderly patients seems to be acceptable, indicating that advanced age does not preclude their administration. In general, dose reductions of mAbs are not recommended, but dose delay is the primary method for the management of side effects (Table 5). Currently available data suggest that renal impairment does not affect the pharmacokinetics of mAbs.60,61 Therefore, we expect that in the case of renal impairment, dose reduction of CD38- and SLAMF7-targeting antibodies will not be necessary. However, until now, no data are available for patients with severely reduced renal function. Importantly, studies with daratumumab and elotuzumab in patients with severe renal impairment are ongoing.
Management and prevention of adverse events associated with elotuzumab and CD38-targeting antibodies
| Adverse event . | Antibody . | Prevention and management . |
|---|---|---|
| General management of adverse events | CD38-targeting antibodies and elotuzumab | In general, dose-delay is the primary method for the management of side effects (and not dose-reductions) |
| Infections | CD38-targeting antibodies and elotuzumab | No formal recommendations can be made at the present time. Herpes zoster prophyaxis should be considered. It is recommended to screen patients for HIV, HBV, and HCV before start of therapy. |
| Infusion-related reactions | Infusion-related reactions occur more frequently with CD38-targeting antibodies than with elotuzumumab | Prevention Premedication, consisting of steroids, antihistamines and acetaminophen, 30-60 minutes prior to infusion.* For patients treated with CD38-targeting antibodies with higher risk of respiratory complications (eg, FEV1 <80%), postinfusion medication should be considered (eg, antihistamines, β-2 adrenergic receptor agonist by inhalation, or control medication for patients with asthma and COPD such as inhalation corticosteroids) For daratumumab: patients with known COPD with a FEV1 <50% of the predicted normal value, with moderate or severe persistent asthma within the past two years, or with uncontrolled asthma, were excluded from trials with daratumumab. Therefore, we recommend to perform FEV1 testing for patients with suspicion of having COPD, and it should be considered to exclude patients from daratumumab treatment if FEV1 <50% of predicted. Given similar pattern and frequency of infusion-related reactions, we also recommend FEV1 testing for patients planned to be treated with isatuximab. Treatment: Interrupt infusion, consider administration of corticosteroids, antihistamines, IV fluid, or β-2 adrenergic receptor agonist by inhalation; after infusion reaction is resolved, restart infusion at lower rate (e.g. half of that used before the interruption) Patients experiencing respiratory events, which occur more frequently with CD38-targeting antibodies, may benefit from pre- and postinfusion prophylaxis with a bronchodilator or in case patients have concomitant asthma or COPD additional medication such as inhalation corticosteroids to control lung disease |
| Adverse event . | Antibody . | Prevention and management . |
|---|---|---|
| General management of adverse events | CD38-targeting antibodies and elotuzumab | In general, dose-delay is the primary method for the management of side effects (and not dose-reductions) |
| Infections | CD38-targeting antibodies and elotuzumab | No formal recommendations can be made at the present time. Herpes zoster prophyaxis should be considered. It is recommended to screen patients for HIV, HBV, and HCV before start of therapy. |
| Infusion-related reactions | Infusion-related reactions occur more frequently with CD38-targeting antibodies than with elotuzumumab | Prevention Premedication, consisting of steroids, antihistamines and acetaminophen, 30-60 minutes prior to infusion.* For patients treated with CD38-targeting antibodies with higher risk of respiratory complications (eg, FEV1 <80%), postinfusion medication should be considered (eg, antihistamines, β-2 adrenergic receptor agonist by inhalation, or control medication for patients with asthma and COPD such as inhalation corticosteroids) For daratumumab: patients with known COPD with a FEV1 <50% of the predicted normal value, with moderate or severe persistent asthma within the past two years, or with uncontrolled asthma, were excluded from trials with daratumumab. Therefore, we recommend to perform FEV1 testing for patients with suspicion of having COPD, and it should be considered to exclude patients from daratumumab treatment if FEV1 <50% of predicted. Given similar pattern and frequency of infusion-related reactions, we also recommend FEV1 testing for patients planned to be treated with isatuximab. Treatment: Interrupt infusion, consider administration of corticosteroids, antihistamines, IV fluid, or β-2 adrenergic receptor agonist by inhalation; after infusion reaction is resolved, restart infusion at lower rate (e.g. half of that used before the interruption) Patients experiencing respiratory events, which occur more frequently with CD38-targeting antibodies, may benefit from pre- and postinfusion prophylaxis with a bronchodilator or in case patients have concomitant asthma or COPD additional medication such as inhalation corticosteroids to control lung disease |
FEV1, forced expiratory volume in 1 second.
Prophylactic treatment with the oral leukotriene antagonist montelukast 10 mg the day before and again on the morning of infusion is considered by some investigators to be a useful adjunct to the standard premedication with acetaminophen, antihistamine, and corticosteroid to prevent airway reactions following the administration of CD38-targeting antibodies.
Infusion-related reactions
Characteristics of infusion-related reactions.
In the phase 1 study with single agent elotuzumab, IRRs occurred more frequently prior to the introduction of premedication.29 Most infusion reactions with elotuzumab were grade 1 or 2 and consisted of pyrexia, chills, nausea, vomiting, flushing, dyspnea, cough, headache, dizziness, rash, and hypertension. In elotuzumab-based combinations, infusion reactions occurred in only 7% to 10% of patients with preinfusion prophylaxis with dexamethasone, antihistamines, and acetaminophen.42,43,50 Across all these studies, most infusion reactions (∼70%) occurred with the first dose of elotumzumab. Only 1% of patients in the ELOQUENT-2 study and no patients in the elotuzumab-bortezomib-dexamethasone study had to discontinue therapy because of infusion reactions.42,43 For patients who tolerated the infusion at 2 mL/min, the flow rate was progressively increased to a maximum of 5 mL/min (infusion time <1 hour) in both studies.
Regarding daratumumab infusions, after antihistamines, acetaminophen, and corticosteroids as premedication, IRRs were recorded in 43% to 71% of patients in the monotherapy trials, with most reactions during the first daratumumab infusion.30,31 Infusion reactions were mainly grade 1 to 2 and characterized by rhinitis, pharyngitis, pyrexia, chills, vomiting, cough, and transient bronchospasm leading to dyspnea.30,31 Analysis of the different infusion regimens suggested that infusion rate is a factor that influences the development of infusion reactions.30 In the GEN501 and Sirius studies, there was no treatment discontinuation because of infusion reactions.30 Duration of the first daratumumab infusion is ∼6 to 7 hours, but this was reduced to 3.3 hours by the third infusion.30
Infusion reactions following administration of isatuximab with preinfusion medication consisting of corticosteroids, antihistamines, and acetaminophen had similar clinical characteristics compared with daratumumab.32 Infusion reactions occurred in 43% of the patients with the first infusion of isatuximab at a dose of ≥10 mg/kg, and they were predominantly grade 1 to 2.32 In the combination of isatuximab with lenalidomide-dexamethasone, infusion-related reactions were observed in 38.7% of patients, predominantly at cycle 1. Grade 3 infusion reactions developed in 2 patients, resulting in treatment discontinuation.47
Management of infusion-related reactions
Adequate and timely management of IRRs is important to prevent the development of more serious toxicity and discontinuation of mAb treatment. MM patients treated with mAbs should receive premedications 30 to 60 minutes before the administration of the therapeutic antibody. We recommend to monitor patients for early signs and symptoms of IRRs, especially during the first infusion. In case an IRR develops, the infusion should be temporarily interrupted, and treatment with extra corticosteroids or antihistamines should be given according to the physician’s discretion. β-2 Adrenergic agonists, administered by inhalation, may be helpful when the patient develops bronchospasm, and intravenous fluids should be administered in the case of hypotension. When the symptoms of the IRR have resolved, the infusion can be restarted at a lower infusion rate than the infusion rate prior to the reaction. After restart, the infusion rate can be increased depending on the patient’s condition. Patients experiencing respiratory events, which occur more frequently with CD38-targeting antibodies, may benefit from pre- and postinfusion prophylaxis with a bronchodilator, or in the case that patients have concomitant asthma or chronic obstructive pulmonary disease (COPD), additional medication, such as inhalation corticosteroids, to control their lung disease. It is also critically important that nurses are provided with knowledge and skills to recognize and manage infusion reactions.
Emerging problems with monoclonal antibodies in MM: laboratory interference
Interference of the therapeutic antibody with several laboratory tests may create problems with response evaluation, plasma cell analysis with multiparametric flow cytometry, and blood compatibility testing. The next section reports on these emerging problems and discusses how to manage them (Table 6).
Management of laboratory interference associated with elotuzumab and CD38-targeting antibodies
| Laboratory test . | Antibody . | Management . |
|---|---|---|
| Interference with serum protein electrophoresis and immunofixation assays | Several therapeutic antibodies | DIRA should be performed when daratumumab-treated patients with IgG-κ M-protein have achieved deep response (M-protein <2 g/L) New assays are in development for elotuzumab, isatuximab and MOR202 |
| Interference with multiparametric flow cytometry | Daratumumab, isatuximab, MOR202, and possibly other therapeutic antibodies | Use of newly developed antibodies for flow cytometry, which bind to different epitopes compared with the therapeutic antibody Application of alternative plasma cell identification markers |
| Interference with blood compatibility testing | CD38-targeting antibodies (also observed with anti-CD44 antibodies) | Denaturation of CD38 from reagent RBCs by dithiothreitol Neutralization of therapeutic antibody with neutralizing antibodies or recombinant soluble CD38 Extensive RBC antigen phenotyping before the patient receives the first infusion of the CD38-targeting antibody or RBC antigen genotyping when the patient has already received treatment with an anti-CD38 antibody or a recent blood transfusion (<3 mo) A wallet card that informs physicians and blood banks of the interference with blood compatibility testing should be provided to all patients treated with CD38-binding antibodies |
| Laboratory test . | Antibody . | Management . |
|---|---|---|
| Interference with serum protein electrophoresis and immunofixation assays | Several therapeutic antibodies | DIRA should be performed when daratumumab-treated patients with IgG-κ M-protein have achieved deep response (M-protein <2 g/L) New assays are in development for elotuzumab, isatuximab and MOR202 |
| Interference with multiparametric flow cytometry | Daratumumab, isatuximab, MOR202, and possibly other therapeutic antibodies | Use of newly developed antibodies for flow cytometry, which bind to different epitopes compared with the therapeutic antibody Application of alternative plasma cell identification markers |
| Interference with blood compatibility testing | CD38-targeting antibodies (also observed with anti-CD44 antibodies) | Denaturation of CD38 from reagent RBCs by dithiothreitol Neutralization of therapeutic antibody with neutralizing antibodies or recombinant soluble CD38 Extensive RBC antigen phenotyping before the patient receives the first infusion of the CD38-targeting antibody or RBC antigen genotyping when the patient has already received treatment with an anti-CD38 antibody or a recent blood transfusion (<3 mo) A wallet card that informs physicians and blood banks of the interference with blood compatibility testing should be provided to all patients treated with CD38-binding antibodies |
DIRA, daratumumab interference reflex assay.
Interference with SPEPs and IFE assays
Characteristics of interference with SPEP and IFE assays.
SPEP is used for the recognition and quantification of M-proteins, whereas IFE is required to determine the M-protein isotype and for confirmation of CR.62,63 Importantly, daratumumab can be detected as an individual monoclonal band in SPEP and serum IFE in the cathodal end of the γ region.30,64 Comigration of daratumumab with the patient’s M-protein may lead to a small absolute overestimation of the concentration of the original M-protein, because pharmacokinetic studies have shown that daratumumab peak plasma concentrations may reach up to 1 g/L. Because daratumumab migrates to the cathodal end of the γ region, it is expected that this mAb preferentially comigrates with IgG M-proteins, whereas other M-protein isotypes migrate more toward the anode.65-67 More importantly, comigration of daratumumab (IgG1-κ mAb) with IgG-κ M-proteins can mask clearance of the endogenous M-protein by serum IFE, which is necessary for documentation of CR. It is expected that ∼50% of IgG-κ M-proteins comigrate with daratumumab, whereas the other M-proteins migrate at a different position than daratumumab and are easily identifiable.67 Finally, in all patients treated with daratumumab, the appearance of a new IgG-κ band may represent daratumumab and should not be misinterpreted as disease recurrence, development of a new plasma cell malignancy, or development of a secondary monoclonal gammopathy of undetermined significance (new monoclonal band on IFE or SPEP that is different from the original M-protein and reflects a strong humoral immune response68 ).
Similarly, elotuzumab could be detected in SPEP (early γ region) and IFE with good specificity and sensitivity in samples from patients treated with elotuzumab, indicating that CR rates could have been underestimated (Figure 2).69,70 Indeed, in the ELOQUENT-2 study, the CR rate was lower in the elotuzumumab group than in the control group (4% vs 7%).42 Similarly, in the randomized phase 2 study evaluating the elotuzumab-bortezomib-dexamethasone combination, CR rates in both groups were similar (4% and 4%). Altogether, this indicates that it is likely that CR rates in elotuzumab studies are underestimated due to interference from therapeutic antibody in SPEP and IFE assays.
Elotuzumab can be detected in SPEP and IFE in samples from patients treated with elotuzumab. (A) Serum IFE detected an IgA-λ M-protein at baseline. (B) After 8 cycles of lenalidomide-dexamethasone and elotuzumab (ELOQUENT-2 study), there was development of a new IgG-κ band of 0 to 2 g/L. In the anti-elotuzumab assay, one of the anti-immunoglobulin antibodies (anti-IgM or anti-IgA) used to precipitate the immunoglobulins was replaced by an anti-elotuzumab antibody (2 mg/mL) with reactivity to an elotuzumab epitope. If elotuzumab is present in patient sera, the anti-idiotypic antibody-elotuzumab complex precipitates, and a band is detected. As elotuzumab is an IgG-κ monoclonal antibody, elotuzumab is precipitated by anti-κ and anti-γ heavy chain antisera, and the 2 bands should align with the elotuzumab band. Indeed, the anti-elotuzumab assay, performed at cycle 34, detected elotuzumab in the IgG-κ band. SIFE, serum immune fixation.
Elotuzumab can be detected in SPEP and IFE in samples from patients treated with elotuzumab. (A) Serum IFE detected an IgA-λ M-protein at baseline. (B) After 8 cycles of lenalidomide-dexamethasone and elotuzumab (ELOQUENT-2 study), there was development of a new IgG-κ band of 0 to 2 g/L. In the anti-elotuzumab assay, one of the anti-immunoglobulin antibodies (anti-IgM or anti-IgA) used to precipitate the immunoglobulins was replaced by an anti-elotuzumab antibody (2 mg/mL) with reactivity to an elotuzumab epitope. If elotuzumab is present in patient sera, the anti-idiotypic antibody-elotuzumab complex precipitates, and a band is detected. As elotuzumab is an IgG-κ monoclonal antibody, elotuzumab is precipitated by anti-κ and anti-γ heavy chain antisera, and the 2 bands should align with the elotuzumab band. Indeed, the anti-elotuzumab assay, performed at cycle 34, detected elotuzumab in the IgG-κ band. SIFE, serum immune fixation.
Other antibodies (eg, ofatumumab, rituximab, siltuximab, trastuzumab, bevacizumab, infliximab, cetuximab, natalizumab, and adalimumab) can also be detected by SPEP and IFE.71-73 The majority of these IgG-κ antibodies electrophorese in the middle of the γ region, whereas rituximab and trastuzumab migrate toward the cathode.71,72 These monoclonal components disappear gradually after completion of antibody therapy.71,73 Clinicians should be aware of potential detection of therapeutic antibodies to prevent additional laboratory testing to exclude an underlying plasma cell disorder in these cases.
Management of interference of therapeutic antibodies with SPEP and IFE assays
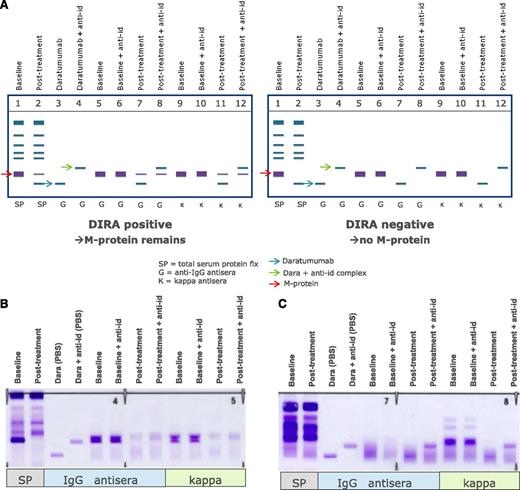
Altogether, this clearly indicates that mitigation strategies are needed to remove interference of the therapeutic antibody to ensure correct response classification. The issue of interference between daratumumab and endogenous M-proteins with overlapping migration in SPEP and IFE tests was addressed by developing DIRA.67 In this assay, interference was mitigated by using a mouse-anti-daratumumab antibody, which binds daratumumab and shifts the migration of daratumab away from the M-protein on immunofixation.64,67 DIRA should be performed in cases where patients with IgG-κ M-protein have achieved a deep response to therapy (M-protein <2 g/L), when SPEP is no longer sensitive enough, to determine whether residual IgG-κ on IFE is caused by daratumumab or endogenous M-protein (Figure 3).64 When DIRA demonstrates complete serologic response, additional testing, including bone marrow examination and free-light chain measurements, should be done to confirm CR or stringent CR.64
Management of interference of daratumumab with SPEP and IFE assays by using DIRA. (A) Schematic representation of DIRA for a DIRA-positive (left) and DIRA-negative case (right). (B) Example from a patient with IgG-κ M protein, who was treated with daratumumab. This patient developed a second IgG-κ band at the end of cycle 1. Response evaluation at start of cycle 16 demonstrated a decrease in M-protein from 31 to 0 g/L by SPEP analysis. Therefore, the DIRA assay was performed, which was positive, indicating that this patient had achieved a very good partial response. (C) Example from a patient with κ light chain MM, who was treated with daratumumab. This patient developed an IgG-κ band of 0 to 1 g/L at the end of the first cycle. The DIRA assay was performed at cycle 11 because of normalization of κ light chains with normal free light chain ratio. The DIRA assay was negative, which triggered bone marrow examination and showed no clonal plasma cells. Therefore, this patient had achieved stringent CR. The new IgG-κ band, which shifted after adding the anti-daratumumab antibody, represents daratumumab. anti-id, anti-idiotype.
Management of interference of daratumumab with SPEP and IFE assays by using DIRA. (A) Schematic representation of DIRA for a DIRA-positive (left) and DIRA-negative case (right). (B) Example from a patient with IgG-κ M protein, who was treated with daratumumab. This patient developed a second IgG-κ band at the end of cycle 1. Response evaluation at start of cycle 16 demonstrated a decrease in M-protein from 31 to 0 g/L by SPEP analysis. Therefore, the DIRA assay was performed, which was positive, indicating that this patient had achieved a very good partial response. (C) Example from a patient with κ light chain MM, who was treated with daratumumab. This patient developed an IgG-κ band of 0 to 1 g/L at the end of the first cycle. The DIRA assay was performed at cycle 11 because of normalization of κ light chains with normal free light chain ratio. The DIRA assay was negative, which triggered bone marrow examination and showed no clonal plasma cells. Therefore, this patient had achieved stringent CR. The new IgG-κ band, which shifted after adding the anti-daratumumab antibody, represents daratumumab. anti-id, anti-idiotype.
A similar shift assay was developed for siltuximab,71 and new assays are under development for elotuzumab, isatuximab, and MOR202 (written communication: Dr E. Bleickardt, BMS, September 29, 2015; Dr S. Janssen, Sanofi, September 29, 2015; and Dr S. Haertle, MorphoSys, September 24, 2015). It is important to provide the laboratory with information when a MM patient receives antibody-based therapy.
Interference with plasma cell identification by multiparametric flow cytometry
Flow cytometry is used for the quantitation of normal and neoplastic plasma cells at presentation and follow-up, including MRD monitoring.74-81 This requires optimized combinations of phenotypic markers to reach high specificity and sensitivity. In antibody panels, CD38 is used as a surface marker combined with CD138 to enumerate the whole plasma cell compartment, whereas markers such as CD19, CD27, CD45, CD56, CD81, and CD117 in combination with cytoplasmic immunoglobulin κ and λ light chains are used to discriminate between normal and clonal plasma cells.78,82
CD38 has emerged as a highly valuable plasma cell identification marker.82 However, daratumumab affects the availability of the epitope for binding of many commercially available CD38 antibodies, which makes the usage of CD38 unreliable for plasma cell identification in patients treated with CD38-targeting antibodies (Figure 4). Furthermore, during therapy with daratumumab, there is a significant reduction of CD38 expression on residual MM cells, which persists for ∼6 months after the last infusion.57 Similarly, isatuximab83 and MOR202 (Dr S. Haertle, MorphoSys, written communication, September 24, 2015) also interfere with binding of some commercially available CD38 antibodies. CD138-targeting antibodies and elotuzumab may also hamper the use of CD138 and CD319 as markers for the identification of plasma cells.
Daratumumab masks the detection of CD38 with commercially available anti-CD38 flow cytometry antibodies. (A) Representative dot plots showing expression of CD38 on BM-localized MM cells (blue) by using a commercially available anti-CD38 flow cytometry antibody before start of daratumumab therapy (left dot plot) and after 10 infusions of daratumumab (right dot plot). The right dot plot demonstrates that, in daratumumab-treated patients, the CD38 antigen cannot be detected by flow cytometry with commercially available anti-CD38 antibodies due to epitope occupancy of the therapeutic antibody. (B) Dot plots from the same patient showing expression of CD38 on BM-localized MM cells (blue) by using the newly developed anti-CD38 monoclonal antibody, HuMax-003, which binds to a different epitope compared with daratumumab. This excludes the possibility that binding of daratumumab masks the detection of CD38. The left dot plot is obtained before start of daratumumab therapy and the right dot plot after 10 daratumumab infusions. These dot plots also illustrate that during daratumumab treatment, there is significant reduction of CD38 expression on residual MM cells.
Daratumumab masks the detection of CD38 with commercially available anti-CD38 flow cytometry antibodies. (A) Representative dot plots showing expression of CD38 on BM-localized MM cells (blue) by using a commercially available anti-CD38 flow cytometry antibody before start of daratumumab therapy (left dot plot) and after 10 infusions of daratumumab (right dot plot). The right dot plot demonstrates that, in daratumumab-treated patients, the CD38 antigen cannot be detected by flow cytometry with commercially available anti-CD38 antibodies due to epitope occupancy of the therapeutic antibody. (B) Dot plots from the same patient showing expression of CD38 on BM-localized MM cells (blue) by using the newly developed anti-CD38 monoclonal antibody, HuMax-003, which binds to a different epitope compared with daratumumab. This excludes the possibility that binding of daratumumab masks the detection of CD38. The left dot plot is obtained before start of daratumumab therapy and the right dot plot after 10 daratumumab infusions. These dot plots also illustrate that during daratumumab treatment, there is significant reduction of CD38 expression on residual MM cells.
Importantly, HuMax-003, a recently developed CD38 mAb, binds to a different epitope compared with daratumumab, excluding the possibility that prior binding of daratumumab masks the detection of CD38. Thus, HuMax-003 may emerge as a useful reagent as part of a MM flow panel to analyze CD38 expression in daratumumab-treated patients (Figure 4). Similarly, the CD38 antibody proposed in the EuroFlow-IMF antibody panel for MRD detection is not hindered by isatuximab or daratumumab (Dr A. Orfao, written communication, August 29, 2015). Alternatively, new plasma cell identification markers, such as CD229, CD269 (BCMA), and CD319 (SLAMF7),82,84 may be used as a substitute for CD38 in flow cytometric analysis. Molecular assessment of MRD, such as next-generation sequencing, is not hampered by therapeutic antibodies.
Interference with blood compatibility testing
Characteristics of interference with blood compatibility testing.
Because CD38 is also weakly expressed on human erythrocytes, therapeutic CD38-targeting antibodies interfere with routine pretransfusion laboratory tests.85-88 The issue of interference and strategies to overcome the interference with blood compatibility testing has been studied in detail in the daratumumab trials30,85,86 and will be discussed here. Importantly, interference is not restricted to CD38-targeting antibodies, but has also been observed for CD44 antibodies (N. Som, transfusion laboratory, VUmc, written communication, September 1, 2015).
Although daratumumab does not interfere with ABO/RhD typing,86 the plasma of daratumumab-treated patients is panreactive in routine serologic tests as a result of binding of daratumumab to CD38 on reagent erythrocytes.85,86 This includes positive antibody screens and panreactive plasma in erythrocyte panel testing (positive indirect antiglobulin test).85,86 Reactions remained positive for 2 to 6 months after the last daratumumab infusion.85 Because of the interference of daratumumab with the indirect antiglobulin test, detection of irregular antibodies in the patients’s plasma is masked for up to 6 months after the last daratumumab infusion (Figure 5A-B). This interference can prevent transfusion laboratories from completing routine pretransfusion testing, and therefore it complicates the selection of suitable blood products for daratumumab-treated patients.85,86 However, until now, no major transfusion-related events were observed when daratumumab-treated patients received a blood transfusion.85 Importantly, interference with routine laboratory tests used in blood transfusion medicine is a class effect of the CD38-targeting antibodies, because red blood cell agglutination was also observed with isatuximab and MOR202.85
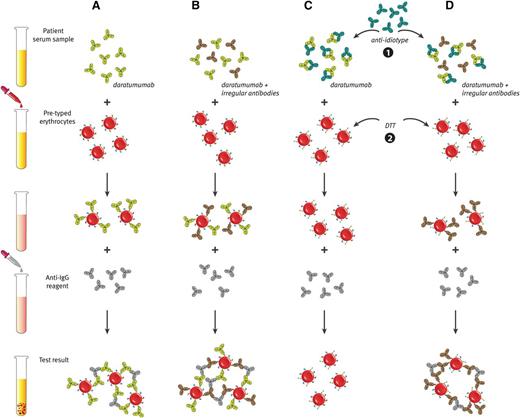
Daratumumab interferes with blood compatibility testing. (A) The plasma of daratumumab-treated patients is panreactive in routine serologic tests as a result of binding of daratumumab to endogenous CD38 on reagent red blood cells. This causes positive antibody screen tests and panreactive plasma in erythrocyte panel testing (indirect antiglobulin test). (B) Therefore, daratumumab interferes with the detection of irregular antibodies, which complicates the selection of suitable blood products for the transfusion of daratumumab-treated patients. (C and D) Interference of daratumumab in blood compatibility testing can be abrogated by neutralizing daratumumab in plasma samples from daratumumab-treated patients with (1) an anti-idiotype antibody (mouse-anti-daratumumab antibody) or (2) by denaturation of surface CD38 from reagent red blood cells by using the reducing agent dithiothreitol (DTT). (D) With both methods, underlying allo-antibodies against red blood cells can be identified in the presence of daratumumab.
Daratumumab interferes with blood compatibility testing. (A) The plasma of daratumumab-treated patients is panreactive in routine serologic tests as a result of binding of daratumumab to endogenous CD38 on reagent red blood cells. This causes positive antibody screen tests and panreactive plasma in erythrocyte panel testing (indirect antiglobulin test). (B) Therefore, daratumumab interferes with the detection of irregular antibodies, which complicates the selection of suitable blood products for the transfusion of daratumumab-treated patients. (C and D) Interference of daratumumab in blood compatibility testing can be abrogated by neutralizing daratumumab in plasma samples from daratumumab-treated patients with (1) an anti-idiotype antibody (mouse-anti-daratumumab antibody) or (2) by denaturation of surface CD38 from reagent red blood cells by using the reducing agent dithiothreitol (DTT). (D) With both methods, underlying allo-antibodies against red blood cells can be identified in the presence of daratumumab.
Management of interference with blood compatibility testing
Standard transfusion laboratory techniques, including multiple rounds of adsorption with untreated or ZZAP (mixture of cysteine-activated proteolytic papain and dithiothreitol)-treated red blood cells (RBCs), failed to remove the panreactivity from the plasma of daratumumab-treated patients.86 However, 2 potential methods to negate daratumumab interference in blood compatibility testing are in development, which can be used in transfusion laboratories to prevent potential blood transfusion problems (Figure 5C-D).
A recent study showed that daratumumab interference in pretransfusion tests could be negated by denaturation of surface CD38 from reagent RBCs by using the reducing agent dithiothreitol.86 This allows the identification of underlying clinically significant alloantibodies against RBCs in the presence of daratumumab.86 An important disadvantage of this technique is the disruption of other dithiothreitol-sensitive blood group systems, including KEL, YT, and DO.86 Missing alloantibodies against these blood group antigens may lead to clinically significant hemolytic reactions.
The second approach is the inhibition of daratumumab binding to CD38 on erythrocytes by neutralizing daratumumab in plasma samples from daratumumab-treated patients with a mouse-anti-daratumumab antibody.85,86 This eliminates the panreactivity in antibody screens and other pretransfusion tests of daratumumab-containing plasma and allows the identification of clinically significant alloantibodies and safe release of blood products for these patients.85,86 Recombinant soluble CD38 (rsCD38) also blocks interference by daratumumab and may therefore be useful as alternative to mouse-anti-daratumumab.85 Application of rsCD38 may also negate interference of other CD38-targeting antibodies in blood compatibility testing.85
Denaturation of CD38 by dithiothreitol has its limitations and, although daratumumab neutralization is a simple technique, at this moment, mouse-anti-daratumumab antibody and rsCD38 are not widely available. Furthermore, additional clinical validation of these assays is needed. Therefore, we currently recommend to perform extensive RBC antigen phenotyping before the patient receives the first infusion of daratumumab or another CD38-targeting antibody.85 RBC genotyping is an alternative to phenotyping, but associated with higher costs. After transfusion of donor erythrocytes, phenotyping is impossible during a period of 3 months, and blood group antigens should be determined by molecular techniques (genotyping). RBC antigen genotyping is also required when the patient has already received treatment with CD38 antibodies. If the patient subsequently requires a blood transfusion in an elective situation, we recommend selection of RBC units that are compatible for clinically relevant blood group antigens (ie, D, C, c, E, and e, and Kell, Kidd, Duffy, and MNS antigens), based on the earlier phenotyping/genotyping results. This will prevent the development of irregular antibodies against these blood groups or hemolytic transfusion reactions in case of presence of such alloantibodies.85 This approach, however, is time-consuming and does not exclude the presence of other irregular antibodies due to the interference of CD38-targeting antibodies in laboratory tests. In case of an acute and life-threatening situation, we recommend selection of ABO/RhD-compatible RBC units that are compatible to as many previously determined other blood group antigens as possible. During transfusion, the patient should be strictly monitored for (hemolytic) transfusion reactions. Blood group O RhD-negative red cells should be issued in emergency situations, where life-saving transfusion is required.
Conclusion and future directions
In summary, the recent development of elotuzumab and selected CD38-targeting antibodies has proven transformative in MM. These mAbs are generally well tolerated and have marked activity either as a single agent and/or in combination with other anti-MM agents in relapsed/refractory MM. Moreover, preliminary results from phase 1 and 2 trials with several other antibodies and antibody-drug conjugates are very encouraging.89-100 Given their unique mechanism of action as well as favorable tolerability, this new class of agents offers tremendous promise in further improving patient outcome in MM. The treatment of MM patients with antibody-based therapies will thus be a valuable approach in the relapsed/refractory setting especially in combination and should prove important for patients with newly diagnosed disease.
As antibodies emerge as important anti-MM agents, a better understanding of adverse events and laboratory interference is critical to optimize their use.
Acknowledgments
The authors thank Victor Muñoz Sanz (Sanz Serif Research+Design Agency) for creating Figure 1 and Jeroen van Velzen and Dr Andries C. Bloem (Department of Immunology, University Medical Center Utrecht, Utrecht, The Netherlands) for help with the selection of flow cytometric images. The authors also thank Dr Kate Sasser and Dr Amy Axel (Janssen Pharmaceuticals) for providing DIRA assay examples, Dr Eric Bleickardt (BMS) for providing examples of elotuzumab interfering with SPEP/IFE, Dr Maarten Janmaat (Genmab) for creating Figure 5, and N. Som (Blood Bank VUmc, Amsterdam, The Netherlands) for useful discussions and comments on the manuscript.
Authorship
Contribution: N.W.C.J.v.d.D. performed literature searches and prepared the first draft of the manuscript; and all other authors reviewed and edited the draft of the report and approved the final manuscript.
Conflict-of-interest disclosure: N.W.C.J.v.d.D. has received research support from Janssen Pharmaceuticals, AMGEN, and Celgene and serves in advisory boards for Janssen Pharmaceuticals, AMGEN, Novartis, and Celgene. P.M. serves on the advisory board and receives honoraria from Janssen Pharmaceuticals. T.P. serves on advisory boards for Genmab and Janssen Pharmaceuticals and has received research support from Janssen Pharmaceuticals. A.P. has consulted for Amgen, Bristol-Myers Squibb, Genmab A/S, Celgene, Janssen-Cilag, Millennium Pharmaceuticals Inc, Onyx Pharmaceuticals and has received honoraria from Amgen, Novartis, Bristol-Myers Squibb, Genmab A/S, Celgene, Janssen-Cilag, Millennium Pharmaceuticals Inc, Onyx Pharmaceuticals, and Sanofi Aventis. F.G. has received honoraria from Celgene and Janssen Pharmaceuticals and serves on advisory boards for Mundipharma, Janssen Pharmaceuticals, and Sanofi. J.P.L. has received grant support from Onyx, Celgene, Novartis, and Millennium. F.M. has received research support and honoraria as speaking fees from Janssen Pharmaceuticals. H.A.-L. declares no competing financial interests. P.S. has received research support and honoraria to the institution from Janssen Pharmaceuticals and honoraria to the institution from BMS and Sanofi. M.-V.M. has received honoraria for the participation on advisory boards of Janssen Pharmaceuticals. H.M.L. has received research support from Celgene, AMGEN, Genmab, and Johnson & Johnson. P.G.R. has served on advisory boards for Millennium Pharmaceuticals, Celgene Corporation, Novartis, Johnson & Johnson, and Bristol Myers Squibb.
Correspondence: Niels W.C.J. van de Donk, Department of Hematology, VU University Medical Center, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: n.vandedonk@vumc.nl.