Abstract
Deep-vein thrombosis (DVT) is regarded a chronic disease as it often recurs. DVT affects most frequently the lower limbs and hence DVT of the leg will be the focus of this article. Whereas algorithms were developed and validated for the diagnosis of a first DVT, no such well-defined strategies exist in the case of recurrence of DVT. Likewise, the scientific evidence regarding the treatment of recurrent DVT is sparse, in particular when it comes to deciding on the duration of anticoagulation. Two typical cases of recurrent DVT, one with an unprovoked DVT and one with DVT during anticoagulation, will be presented. Based on these two clinical scenarios, algorithms for the diagnosis and treatment of recurrent DVT will be put forward. The purpose of this article is to discuss strategies that can be applied in daily clinical practice by physicians who do not have access to means and measures available in specialized thrombosis centers.
Case 1
A 59-year-old man presented for pain and swelling of the left calf. He had been doing well until 3 days earlier when symptoms all of a sudden began. He did not have surgery, an injury to the leg, or a medical illness requiring prolonged bed rest during the previous months. He did report, however, that he had had similar symptoms ∼3 years ago in the same leg after a skiing accident. According to a prior medical record, he had been diagnosed with isolated deep vein thrombosis (DVT) in the posterior tibial veins and was treated with a “blood thinner” for several months. Physical examination revealed tenderness and warmth of the calf, and an increase in calf diameter of 3 cm compared with the other leg. Deep palpation of the calf muscles was painful. Recurrent DVT was suspected. What are the next diagnostic steps?
Diagnosis of recurrent DVT
The diagnosis of recurrent DVT is of particular clinical importance. Many patients in whom such a diagnosis is established will receive extended and sometimes life-long anticoagulant therapy, which means that they will be exposed to a considerable bleeding risk. Conversely, if the diagnosis is missed, untreated patients have a high risk of thrombus progression and embolization. In contrast to the diagnosis of a first DVT of the leg, which follows validated algorithms including a pretest probability assessment, measurement of D-Dimer (DD), and compression ultrasonography (CUS),1 no such strategies are established for the diagnosis of recurrent DVT. This diagnostic ambiguity can be explained by the absence of clinical studies defining a valid clinical end point. A pretest probability score has never been developed for patients with suspected recurrent DVT. Nevertheless, an individual clinical judgment of the likelihood of recurrent thrombosis, taking into account signs and symptoms indicative for DVT as well as strong risk factors of thrombosis such as recent surgery, trauma, prolonged bed rest, or active cancer should always be the first step during the diagnostic work-up. The history of a previous venous thromboembolism (VTE) per se classifies such patients at risk of thrombosis.
The role of DD is less well studied in patients with a recurrent DVT. Incorporating DD in an algorithm to diagnose or exclude recurrent DVT could nevertheless be potentially helpful: none of the 16 untreated patients with a low clinical likelihood of recurrence according to the modified Well’s score and a negative DD had recurrent DVT during a 3-month follow-up.2,3 Only one of 134 patients with a negative DD experienced recurrence during a 3-month follow-up, but recurrence could not be definitively ruled out in another 6 patients.4 A failure rate of only 1% was recorded among almost 1000 untreated patients with suspected recurrent DVT, who had a negative DD and a low pretest probability. DD levels may remain elevated for a long time after a first VTE, thereby possibly deflating the diagnostic utility for the second event.5-8
The mainstay of DVT diagnosis, also in the scenario of recurrence, is imaging. Several methods to detect DVT have been studied including venography, CUS, computed tomography venography, and magnetic resonance direct thrombus imaging. Venography was used as an outcome standard in several accuracy studies of patients with suspected recurrent DVT,9-12 but has never been validated for this purpose. Venography is invasive and can be associated with serious complications. It is often complicated by technical problems and, in the era of CUS, the expertise among radiologists to perform and interpret a venogram has declined. Nowadays, the gold standard for diagnosing a first DVT has become CUS, with a sensitivity for proximal and distal DVT >90% and 60%, respectively, and a specificity of almost 100%.13 Approximately half of the DVT recurrences occur in the so far unaffected contralateral leg.14 There is nothing to assume that in this case CUS would not perform equally as well as in a first DVT. The diagnosis of an ipsilateral recurrent DVT is far more challenging. The finding of non-compressibility of an ipsilateral femoral or popliteal vein segment, which was previously not affected, can be considered diagnostic. Sometimes the initial thrombus does not completely resolve, resulting in residual vein thrombosis.15,16 For diagnosing recurrent DVT in a previously affected vein segment, the criterion of an increase in thrombus diameter of at least 4 mm on (serial) CUS, possibly in combination with a DD measurement, has been put forward.12,17,18 In a validation study, 8 of 284 patients in whom recurrent VTE had been excluded by this approach and who were left untreated had a VTE with a 3-month risk of 2.8% and an upper 95% confidence interval (CI) of 5.5%.17 Its applicability is dependent upon a highly experienced investigator and the availability of a previous CUS result. It also cannot be used to detect recurrent calf vein thrombosis. A guidance panel suggests that proximal CUS should be performed at the time of withdrawal of anticoagulation to obtain a baseline measurement.19 This is however, rarely done in everyday practice.
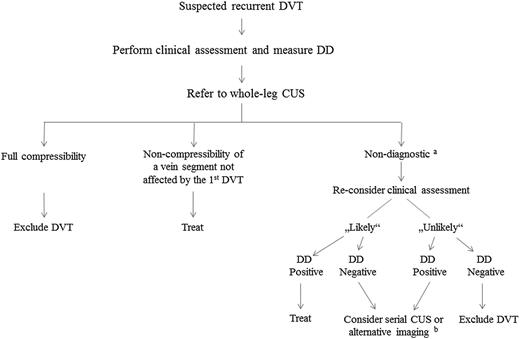
Figure 1 shows an algorithm for the diagnosis of recurrent DVT in routine clinical practice. The first step is to estimate the clinical likelihood of DVT. In the absence of a validated clinical prediction rule, this should be done with a thorough individual clinical judgment looking for symptoms and signs indicative for DVT as well as for important risk factors. The second step is whole-leg CUS. If the deep leg veins are fully compressible, DVT is ruled out. In case of noncompressibility of a vein segment not affected by the first DVT, the diagnosis is established. If CUS is nondiagnostic (ie, noncompressibility of a previously affected vein segment or noncompressibility of any vein segment in the ipsilateral leg in the absence of a previous CUS result), a strategy combining clinical assessment and DD is followed. For patients in whom the diagnosis is still ambiguous, serial CUS or alternative imaging tests can be considered although they rarely offer an additional diagnostic certainty.
Suggested procedure to diagnose recurrent DVT.aNoncompressibility of a previously affected vein segment or noncompressibility of any vein segment in the ipsilateral leg in the absence of a previous CUS result; balternative imaging techniques include venography, computed tomography venography, and magnetic resonance direct thrombus imaging.
Suggested procedure to diagnose recurrent DVT.aNoncompressibility of a previously affected vein segment or noncompressibility of any vein segment in the ipsilateral leg in the absence of a previous CUS result; balternative imaging techniques include venography, computed tomography venography, and magnetic resonance direct thrombus imaging.
Case 1 (continued)
The clinical likelihood of recurrent DVT was considered high. On whole-leg CUS, noncompressibility of the femoral and the popliteal vein was found. Upon the basis of noncompressibility of a previously unaffected vein segment, the diagnosis of recurrent DVT was made. The positive DD supported the presence of a thrombosis, but had no part in deciding on the diagnostic work-up. What are the treatment options for a patient with a recurrent DVT?
Anticoagulant treatment of recurrent DVT
How to estimate the bleeding risk?
Deciding on the mode and duration of anticoagulation entails balancing the risk of recurrent thromboembolism against the risk of bleeding. It is, therefore, mandatory to evaluate the bleeding risk not only before anticoagulation is installed but also at regular intervals thereafter. Scoring systems to estimate the bleeding risk exist for patients with venous thrombosis,20-22 but, unfortunately, performs poorly,23-25 is not validated, and cannot be recommended for use in daily practice. The annual long-term risk of major bleeding during anticoagulation ranges between <1% and >6.5%, and is dependent upon the presence or absence of various risk factors, including advanced age, previous bleeding, cancer, thrombocytopenia, antiplatelet therapy, recent surgery, previous stroke, or other comorbidities.26 These bleeding risk percentages may not be valid for patients with a second DVT who already tolerated prior anticoagulation well. Ultimately, the estimation of the bleeding risk relies on the discretion of the treating physician rather than on scientific evidence.
Which anticoagulant regimen should be used for acute treatment?
Data on the treatment of acute recurrent DVT is scarce. Only a small number of patients with a history of previous VTE were included in the large treatment trials. It thus remains that considerations and treatment options for a recurrent acute DVT will not be different to that of a first event, and immediate and intensive anticoagulation is mandatory. Unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux followed by a vitamin K antagonist (VKA) were the only treatment options until recently.27 The field of anticoagulation has lately changed with the appearance of the direct oral anticoagulants (DOACs). Four DOACs (rivaroxaban, apixaban, and edoxaban are direct factor Xa inhibitors; dabigatran is a direct thrombin inhibitor) gained approval in many countries worldwide. DOACs are equally effective as heparin followed by a VKA but confer a lower risk of major and fatal bleeding.28-30 Dabigatran and edoxaban are started after a lead-in phase with heparin. Rivaroxaban and apixaban are administered as all-oral regimens with a higher dose at the beginning.
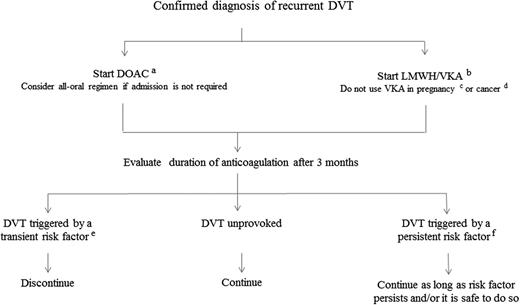
Figure 2 suggests an algorithm for the treatment of recurrent DVT in routine clinical practice. In the absence of a contraindication, anticoagulation is started as soon as possible. If the patient does not need to be admitted or can be discharged early, an all-oral regimen with a DOAC is more convenient. In patients in whom a DOAC is not given, LMWH at a therapeutic dose followed by a VKA is an alternative. Pregnant women must not be treated with a DOAC but should receive LMWH at a therapeutic dose. In patients with cancer, treatment with a DOAC is seen controversially and guidelines recommend against its use.31,32 Consequently, cancer patients with a recurrent DVT should receive LMWH at a therapeutic dose. Thrombolysis or thrombectomy can be considered in younger patients with extensive DVT, who have a low bleeding.
Suggested treatment protocol for recurrent DVT.aApixaban 10 mg twice daily for 1 week followed by 5 mg twice daily, reduce dose to 2.5 mg twice daily after 6 months; rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg once daily; LMWH once or twice daily at therapeutic dose for at least 5 days followed by 150 mg dabigatran twice daily or by 60 mg edoxaban once daily; bLMWH once or twice daily at therapeutic dose together with a VKA (target INR, 2.0-3.0) and continue LMWH until a stable INR has been reached, but for a minimum of 5 days; cLMWH at therapeutic dose until 24 hours before induction of labor or caesarean section, and restart LMWH at a reduced dose; dLMWH at therapeutic dose, reduced to about 75% at 4 weeks for at least 6 months or as long as it is safe to do so; etransient risk factors include surgery, trauma, prolonged bed rest, oral contraceptives, hormone replacement therapy, pregnancy/puerperium; and fpersistent risk factors include inflammatory bowel disease, antiphospholipid syndrome, nephrotic syndrome, paroxysmal nocturnal hemoglobinuria, myeloproliferative neoplasma, Behçet syndrome, postthrombotic syndrome, and congenital venous malformation.
Suggested treatment protocol for recurrent DVT.aApixaban 10 mg twice daily for 1 week followed by 5 mg twice daily, reduce dose to 2.5 mg twice daily after 6 months; rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg once daily; LMWH once or twice daily at therapeutic dose for at least 5 days followed by 150 mg dabigatran twice daily or by 60 mg edoxaban once daily; bLMWH once or twice daily at therapeutic dose together with a VKA (target INR, 2.0-3.0) and continue LMWH until a stable INR has been reached, but for a minimum of 5 days; cLMWH at therapeutic dose until 24 hours before induction of labor or caesarean section, and restart LMWH at a reduced dose; dLMWH at therapeutic dose, reduced to about 75% at 4 weeks for at least 6 months or as long as it is safe to do so; etransient risk factors include surgery, trauma, prolonged bed rest, oral contraceptives, hormone replacement therapy, pregnancy/puerperium; and fpersistent risk factors include inflammatory bowel disease, antiphospholipid syndrome, nephrotic syndrome, paroxysmal nocturnal hemoglobinuria, myeloproliferative neoplasma, Behçet syndrome, postthrombotic syndrome, and congenital venous malformation.
What is the optimal duration of anticoagulation?
The duration of anticoagulation is largely dependent upon the risk of recurrent VTE. In contrast to patients with a first VTE, the recurrence risk is less well studied in patients with a second event. Patients with a thrombosis history usually receive thromboprophylaxis when they are later exposed to transient risk conditions such as surgery, trauma, hospitalization, or pregnancy. Provoked recurrences, ie, events that occur in association with a transient risk factor are, therefore, seen infrequently. In the Austrian Study of Recurrent Venous Thrombosis, only 10% of VTE recurrences were rated as “provoked.” In the absence of clinical studies, the recurrence risk can only be extrapolated from patients with a first provoked DVT. There is evidence that patients with a first provoked DVT have a lower recurrence risk than patients with a first unprovoked event. Surgical patients have by far the lowest recurrence risk.33 It is, therefore, justified to treat patients with recurrent DVT associated with a transient risk factor (surgical or nonsurgical) for only a limited period of time (3 to 6 months) because the thrombosis risk in this patient population is outweighed by the risk of bleeding associated with long-term anticoagulation. In patients where the DVT recurred in the presence of a persistent risk factor such as active cancer, autoimmune disease, or inflammatory bowel disease, long-term anticoagulation is reasonable as long as the bleeding risk does not increase (Figure 2).
Patients in whom the first VTE was unprovoked (ie, occurred in the absence of a transient risk factor) have a recurrence risk as high as 30% over 5 years after discontinuation of anticoagulation.34-36 There is circumstantial evidence that a second episode of unprovoked VTE inflicts 1.5 times the risk of recurrent VTE relative to a first episode of unprovoked VTE, resulting in a calculated recurrence risk of almost 50% over 5 years.26 The Duration of the Anticoagulant Trial Study Group performed the only randomized controlled trial comparing short-term oral anticoagulant therapy with warfarin with long-term anticoagulation.37 The majority of patients had an unprovoked VTE as the second event. After a mean follow-up of almost 4 years, patients treated for 6 months had a much higher incidence of recurrence than patients treated infinitely (20.7% vs 2.6%). One major bleed occurred in the short-term treatment group, and 2 fatal bleeds and 8 major bleeds in the long-term treatment group. Taken together, the recurrence risk among patients with an unprovoked second DVT has to be considered as very high, although this notion is largely based upon indirect evidence. Nevertheless, it is commonly agreed upon that these patients should receive long-term anticoagulation. The presence of a postthrombotic syndrome would strengthen this decision even more as DVT recurrence in the same leg would further impair venous flow. Before deciding on long-term anticoagulant treatment, the patient’s preferences and concerns need to be discussed because this decision may have major life-style implications. Once therapy has begun, patients have to be followed-up at regular intervals to evaluate the quality of anticoagulation and the adherence to treatment, and also to capture the appearance of new risk factors of bleeding, which could necessitate interruption or discontinuation of anticoagulation.
Which anticoagulant regimen should be used for long-term treatment?
Until recently, VKA were regarded as first choice for long-term treatment of VTE. Lately, several DOACs were investigated also for extended anticoagulation. Approximately two-thirds of patients included in these trials had a first DVT. Table 1 provides an overview of 3 trials comparing rivaroxaban, dabigatran, and apixaban with placebo for extended VTE prevention after an initial course of 6 months anticoagulation.38-40 The intended treatment durations ranged from 6 to 12 months. Apixaban was given at a therapeutic dose (ie, at the same dose that was used for the treatment of acute VTE) and also at a lower dose. Rivaroxaban and dabigatran were given at a therapeutic dose. DOACs conferred substantial risk reductions ranging between 64% and 92% compared with placebo. Fatal bleeding occurred in none of the patients and major or clinical relevant nonmajor bleeding was infrequent. The incidence of bleeding was not higher in patients treated with apixaban than in the placebo group. RE-MEDY compared dabigatran at a therapeutic dose with warfarin in patients who have completed at least 3 months of anticoagulation.40 The duration of treatment (up to 36 months) was longer than in the other studies. Recurrent VTE was recorded in 1.8% of patients assigned to dabigatran and in 1.3% of warfarin-treated patients for an HR of 1.4 (95% CI, 0.8-2.6). Major bleeding and major or clinically relevant nonmajor bleeding were less frequent among patients treated with dabigatran (0.9% vs 1.8%; HR 0.5, 90% CI, 0.3 to 1.0 and 5.6% vs 10.2%; HR 0.5, 95% CI, 0.4-0.7, respectively). Therefore, in the setting of extended anticoagulation, DOACs effectively prevent recurrent VTE at an acceptable bleeding risk. Some caveats need to be mentioned. The treatment duration of all studies was limited and it is unknown if patients who received long-term anticoagulation benefited to the same extent. The majority of patients included in these trials experienced only one thrombotic event, and it remains to be seen if DOACs are also as effective and safe in patients with multiple events. DOACs are partly cleared from the circulation via the kidneys. Caution should, therefore, be exercised in prescribing DOACs to patients with a creatinine clearance <30 mL/min in the case of the factor Xa inhibitors or 50 mL/min when dabigatran is chosen. Routine monitoring of kidney function at least once or twice a year is prudent, particularly in older patients. Nevertheless, DOACs are an appealing alternative to VKA, also in the setting of long lasting DVT therapy (Figure 2).
Double-blind randomized trials comparing a DOAC with placebo in patients with proximal DVT and/or PE
| Study . | Drug . | Dose (mg) . | N . | Intended treatment duration (mo) . | Recurrent VTE % vs placebo; HR (95% CI) . | Major bleeding % vs placebo; HR (95% CI) . | Major or CRNM bleeding % vs placebo; HR (95% CI) . |
|---|---|---|---|---|---|---|---|
| EINSTEIN extension | Rivaroxaban | 20 OD | 1196 | 6-12 | 1.3 vs 7.1 | 0.7 vs 0 | 6 vs 1.2 |
| 0.18 (0.09-0.39) | 5.19 (2.3-11.7) | ||||||
| RE-SONATE | Dabigatran | 150 BID | 1343 | 6-12 | 0.4 vs 5.6 | 0.3 vs 0 | 5.3 vs 1.8 |
| 0.08 (0.02-0.25) | 2.92 (1.52-5.60) | ||||||
| AMPLIFY extension | Apixaban | 2.5 BID | 2486 | 12 | 3.8 vs 11.6 | 0.2 vs 0.5 | 3.2 vs 2.7 |
| 5 BID | 0.33 (0.22-0.48) | 0.49 (0.09-2.64) | 1.20 (0.69-2.10) | ||||
| 4.2 vs 11.6 | 0.1 vs 0.5 | 4.3 vs 2.7 | |||||
| 0.36 (0.25-0.53) | 0.25 (0.03-2.24) | 1.62 (0.96-2.73) |
| Study . | Drug . | Dose (mg) . | N . | Intended treatment duration (mo) . | Recurrent VTE % vs placebo; HR (95% CI) . | Major bleeding % vs placebo; HR (95% CI) . | Major or CRNM bleeding % vs placebo; HR (95% CI) . |
|---|---|---|---|---|---|---|---|
| EINSTEIN extension | Rivaroxaban | 20 OD | 1196 | 6-12 | 1.3 vs 7.1 | 0.7 vs 0 | 6 vs 1.2 |
| 0.18 (0.09-0.39) | 5.19 (2.3-11.7) | ||||||
| RE-SONATE | Dabigatran | 150 BID | 1343 | 6-12 | 0.4 vs 5.6 | 0.3 vs 0 | 5.3 vs 1.8 |
| 0.08 (0.02-0.25) | 2.92 (1.52-5.60) | ||||||
| AMPLIFY extension | Apixaban | 2.5 BID | 2486 | 12 | 3.8 vs 11.6 | 0.2 vs 0.5 | 3.2 vs 2.7 |
| 5 BID | 0.33 (0.22-0.48) | 0.49 (0.09-2.64) | 1.20 (0.69-2.10) | ||||
| 4.2 vs 11.6 | 0.1 vs 0.5 | 4.3 vs 2.7 | |||||
| 0.36 (0.25-0.53) | 0.25 (0.03-2.24) | 1.62 (0.96-2.73) |
BID, twice daily; CRNM, clinically relevant nonmajor; HR, hazard ratio; OD, once daily.
Case 1 (continued)
The patient was otherwise healthy and risk factors of bleeding were absent. Because there was no need for admission, an all-oral DOAC regimen was started. The patient consented to long-term anticoagulant therapy after having been informed that due to the fact that his second episode was unprovoked, his risk of recurrent DVT is high and most likely outweighs the risk of bleeding. He was advised to take his medication regularly and that missing doses could result in thrombus progression, embolization, or recurrence. Appointments were arranged for the time point of dose reduction and for regular check-ups thereafter.
Case 2
A 27-year-old male presented with swelling and pain of the right leg. Physical examination revealed warmth, edema from the distal thigh downward to the ankle, and tenderness on palpation of the calf. He is a musician and is regularly on tour for long periods, often traveling by plane over long distances. The DD was elevated and CUS showed femoral vein thrombosis. He had already been diagnosed with unprovoked proximal DVT of the left leg 1 year earlier and long-term anticoagulation had been recommended. At the time of presentation he was still on VKA treatment.
Recurrent DVT during anticoagulant therapy
What could be the reason for a recurrent DVT during anticoagulation?
Anticoagulants, when given at a therapeutic dose, are highly effective in preventing recurrence. In the Duration of the Anticoagulant Trial Study Group trial, none of the patients with a second VTE who were given warfarin for up to 4 years had another event during anticoagulation.37 DVT during anticoagulant therapy is, therefore, rare and most disturbing for both patients and physicians. The most likely explanation in patients treated with a VKA or a DOAC alike is insufficient intensity of anticoagulation, most often because of nonadherence to the medication. The half-life of DOACs is short and missing doses may increase the susceptibility for recurrence. In a patient who has recurrence while treated with a VKA, the first step is to assess the quality of treatment by checking the international normalized ratio (INR) determined at the time of recurrence, as well as those measured earlier. In rare cases, the INR is within the therapeutic range. One explanation for a DVT at therapeutic INR could be that a patient who had been incompliant with VKAs developed symptoms of DVT and restarted VKAs, which then led to a therapeutic INR when seeking medical attention. Alternatively, in such a situation, hypercoagulability as the consequence of a risk factor potent enough to overcome the usual intensity of anticoagulation is often suspected. Indeed, cancer patients were found to have a more than threefold higher risk of recurrent VTE than non-cancer patients and more than 80% of events occurred at an anticoagulation level within or above the therapeutic range.41 According to an international registry, 80% of cancer patients who were treated with a VKA for an incident VTE had their “breakthrough” recurrence at an INR >2.42 In a randomized trial of patients with cancer-associated VTE, the probability of recurrent thrombosis at 6 months was 17% in the VKA group and 9% in patients treated with a LMWH.43 Thus, the finding of failures of VKA anticoagulation in patients with cancer infers that DVT recurrence in a patient with a therapeutic INR should raise the suspicion for the presence of a thus far unknown hidden malignancy. Other conditions may be considered as predisposing for a DVT recurrence during adequate anticoagulation, although there is no scientific evidence for this assumption, are anatomical abnormalities such as May-Thurner syndrome, blood diseases including myeloproliferative neoplasms and paroxysmal nocturnal hemoglobinuria, or phospholipid antibodies (Table 2). Notably, in some patients with a phospholipid antibody syndrome, recurrence could be explained by under-anticoagulation due to a falsely therapeutic (or even supra-therapeutic) INR resulting from interference of a lupus anticoagulant with the INR test system.44 In case of prolongation of the baseline PT by a lupus anticoagulant, which causes difficulty in establishing the true degree of anticoagulation, amidolytic factor X assays can be used aiming at a therapeutic factor Xa range of 20% to 40%.45-47 In patients who develop recurrent DVT while on heparin, heparin-induced thrombocytopenia should be suspected.
Possible underlying conditions for recurrent VTE during anticoagulation
| Possible conditions . |
|---|
| Insufficient intensity of anticoagulation Active cancer |
| Anatomical abnormalities (ie, May-Thurner syndrome)* |
| Myeloproliferative neoplasms (ie, polycythemia vera, essential thrombocythemia)* |
| Paroxysmal nocturnal hemoglobinuria* |
| Phospholipid antibody syndrome* |
| Heparin-induced thrombocytopenia |
| Possible conditions . |
|---|
| Insufficient intensity of anticoagulation Active cancer |
| Anatomical abnormalities (ie, May-Thurner syndrome)* |
| Myeloproliferative neoplasms (ie, polycythemia vera, essential thrombocythemia)* |
| Paroxysmal nocturnal hemoglobinuria* |
| Phospholipid antibody syndrome* |
| Heparin-induced thrombocytopenia |
Low level of scientific evidence.
How should a recurrent DVT, which occurred during anticoagulation be treated?
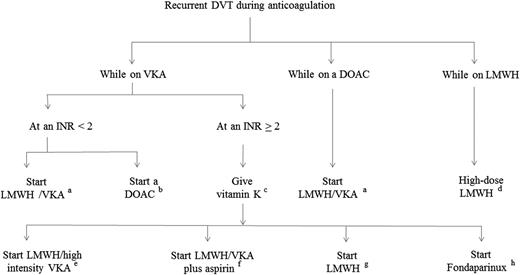
An algorithm for the treatment of patients with recurrent DVT during anticoagulant treatment is shown in Figure 3. In case of a sub-therapeutic INR, the patient should be immediately started on full-dose LMWH. If VKAs are considered for long-term treatment again, all efforts to improve the quality of anticoagulation should be made. These include intense counseling, INR monitoring at closer intervals, or inclusion in a self-monitoring or self-management program. In case the patient is dissatisfied with the VKA treatment, switching to a DOAC may be reasonable. In case of an INR ≥2, vitamin K followed by full-dose LMWH should be given. The presence of a hidden cancer needs to be taken into consideration. Patients, in whom such a diagnosis is suspected or even established, should continue full-dose LMWH. If a cancer patient recurs with DVT despite full-dose LMWH treatment, an increase in the LMWH dose by 25% is suggested.48 There are several therapeutic alternatives if active cancer is unlikely, however, none of them have been studied in greater detail: long-term LMWH at a therapeutic dose, LMWH followed by a VKA with either an upward adjustment of the therapeutic INR range or combined with aspirin, or fondaparinux at a therapeutic dose. In case of recurrence during anticoagulation with one of the DOACs, anticoagulation with LMWH at full dose followed by a VKA aimed at an INR range of 2 to 3, together with intense counseling regarding adherence to the treatment seems to be the best available option.
Suggested treatment protocol for recurrent DVT during anticoagulant treatment.aLMWH once or twice daily at therapeutic dose together with a VKA (target INR, 2.0-3.0) and continue LMWH until a stable INR has been reached, but for a minimum of 5 days; bapixaban 10 mg twice daily for 1 week followed by 5 mg twice daily; rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg once daily; LMWH once or twice daily at therapeutic dose for at least 5 days followed by 150 mg dabigatran twice daily or by 60 mg edoxaban once daily; c10 mg vitamin K orally or IV; dLMWH dose increase by ∼25%; eLMWH once or twice daily at therapeutic dose together with a VKA (target INR, 2.5-4.0) and continue LMWH until a stable INR has been reached, but for a minimum of 5 days; fLMWH once or twice daily at therapeutic dose together with a VKA (target INR, 2.0-3.0) and aspirin (100 mg once daily), and continue LMWH until a stable INR has been reached but for a minimum of 5 days; gLMWH once or twice daily at therapeutic dose; and hfondaparinux weight-adjusted at a therapeutic dose.
Suggested treatment protocol for recurrent DVT during anticoagulant treatment.aLMWH once or twice daily at therapeutic dose together with a VKA (target INR, 2.0-3.0) and continue LMWH until a stable INR has been reached, but for a minimum of 5 days; bapixaban 10 mg twice daily for 1 week followed by 5 mg twice daily; rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg once daily; LMWH once or twice daily at therapeutic dose for at least 5 days followed by 150 mg dabigatran twice daily or by 60 mg edoxaban once daily; c10 mg vitamin K orally or IV; dLMWH dose increase by ∼25%; eLMWH once or twice daily at therapeutic dose together with a VKA (target INR, 2.5-4.0) and continue LMWH until a stable INR has been reached, but for a minimum of 5 days; fLMWH once or twice daily at therapeutic dose together with a VKA (target INR, 2.0-3.0) and aspirin (100 mg once daily), and continue LMWH until a stable INR has been reached but for a minimum of 5 days; gLMWH once or twice daily at therapeutic dose; and hfondaparinux weight-adjusted at a therapeutic dose.
A permanent vena cava filter in addition to anticoagulant therapy appears to be, at first glance, a promising tool to protect patients from recurrent pulmonary embolism (PE). There are, however, several arguments against this approach. In a randomized trial in patients with proximal DVT, vena cava filters together with standard anticoagulation reduced the risk of PE, but increased that of DVT and did not confer a survival benefit.49 DVT patients have more often DVT than PE as the recurrent event,50 and will therefore benefit from filter insertion to a much lesser extent than PE patients. Most importantly, there are safety concerns regarding filter embolization, fracturing and device migration, which have led to a Food and Drug Administration safety alert.51,52 As a consequence, vena cava filters should be used with great caution in patients with (recurrent) DVT and, if at all, only in patients with a very high risk of PE, such as in patients who have a contraindication against anticoagulation.
Case 2 (continued)
At presentation the INR was 1.4. The patient had stopped INR monitoring several months before because of interference with his professional commitments. He refused to take VKA any longer. He was once again counseled with regard to his high thrombosis risk, started on an all-oral DOAC regimen, and instructed that missing doses could result in thrombus progression or recurrence.
Conclusion
The diagnosis of recurrent DVT can be challenging, in particular if it is suspected in the same leg as the first event. In this article a diagnostic strategy is proposed, which can be applied to routine daily practice without knowing the diagnostic details of the previous event. The treatment of acute recurrent DVT is not different from that of a first DVT. Many patients with a second DVT have to be regarded at high risk of another recurrence and are candidates for long-term anticoagulation. DOACs are a reasonable choice for extended anticoagulant therapy because they are convenient to patients and their physicians, are as effective as the VKAs, and confer a lower risk of bleeding. Their performance over a long period of time however, remains unknown.
In conclusion, the scientific evidence for diagnosis and treatment of recurrent DVT is scarce. The algorithms proposed in this article are therefore, in large part, not evidence-based and are likely to change when more data become available.
Authorship
Contribution: P.A.K. wrote the article.
Conflict-of-interest disclosure: P.A.K. received fees for lectures from Boehringer Ingelheim, Bayer Healthcare, and Daiichi Sankyo and served on advisory boards for the same institutions.
Correspondence: Paul A. Kyrle, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria; e-mail: paul.kyrle@meduniwien.ac.at.