Key Points
Heme and iron induce macrophage phenotypic switching toward an M1 proinflammatory phenotype.
By scavenging free heme, hemopexin reverts heme-induced proinflammatory activation of macrophages in a mouse model of sickle cell disease.
Abstract
Hemolytic diseases, such as sickle cell anemia and thalassemia, are characterized by enhanced release of hemoglobin and heme into the circulation, heme-iron loading of reticulo-endothelial system macrophages, and chronic inflammation. Here we show that in addition to activating the vascular endothelium, hemoglobin and heme excess alters the macrophage phenotype in sickle cell disease. We demonstrate that exposure of cultured macrophages to hemolytic aged red blood cells, heme, or iron causes their functional phenotypic change toward a proinflammatory state. In addition, hemolysis and macrophage heme/iron accumulation in a mouse model of sickle disease trigger similar proinflammatory phenotypic alterations in hepatic macrophages. On the mechanistic level, this critically depends on reactive oxygen species production and activation of the Toll-like receptor 4 signaling pathway. We further demonstrate that the heme scavenger hemopexin protects reticulo-endothelial macrophages from heme overload in heme-loaded Hx-null mice and reduces production of cytokines and reactive oxygen species. Importantly, in sickle mice, the administration of human exogenous hemopexin attenuates the inflammatory phenotype of macrophages. Taken together, our data suggest that therapeutic administration of hemopexin is beneficial to counteract heme-driven macrophage-mediated inflammation and its pathophysiologic consequences in sickle cell disease.
Introduction
Several pathologic states are hallmarked by systemic or local release of hemoglobin (Hb) and heme from red blood cells. Sickle cell disease (SCD) and malaria are paradigmatic hemolytic disorders, in which alteration in the structure of red blood cells (RBCs) leads to the release of Hb and heme into the circulation.
Heme is a toxic and pro-oxidant molecule when released in excessive amounts.1-3 To counteract the potentially detrimental effects of Hb and heme, mammals are equipped with extracellular scavenging systems, namely haptoglobin (Hp) and hemopexin (Hx), that bind Hb and heme, respectively.4 Hp-Hb and Hx-heme complexes are taken up by receptor-mediated endocytosis into macrophages and hepatocytes, respectively.1,5-9 Reticulo-endothelial macrophages located in liver and spleen constitute the major sites for the clearance of aged or damaged RBCs. Following RBC phagocytosis or Hp-mediated Hb internalization, Hb is degraded and heme-iron is recycled for de novo erythropoiesis.3 Within cells, heme is catabolized by the activity of heme oxygenases (inducible HO-1 and constitutive HO-2) into iron, carbon monoxide, and biliverdin. The iron is then either bound by the iron storage protein ferritin, to prevent iron-mediated generation of radicals, or exported into the bloodstream by the iron exporter ferroportin (Fpn).10-12 Both RBC clearance and extracellular Hb scavenging are relatively modest events under steady-state conditions, but are drastically enhanced in hemolytic disorders.13,14 High levels of extracellular Hb and heme ultimately lead to the saturation and depletion of the Hp/Hx scavenging systems,15 causing heme-mediated oxidative tissue damage.16
Increasing evidence demonstrates that heme acts as a proinflammatory molecule that binds and activates Toll-like receptor 4 (TLR4), thus inducing the activation of endothelial cells17 and the production of inflammatory cytokines, such as tumor necrosis factor (TNF)α in neutrophils and macrophages.18-22 These observations are interesting in the context of SCD,23-28 malaria,29,30 and other pathologic conditions associated with hemoglobin/heme release,15 such as sepsis,31-34 atherosclerosis,35,36 intracerebral hemorrhage,37,38 and infections,39 in which levels of proinflammatory cytokines are increased. For example, monocytes from SCD patients show an enhanced state of activation, with increased expression of interleukin (IL)-15 and production of TNFα and IL-1β.40
Reticuloendothelial system macrophages are characterized by marked phenotypical and functional heterogeneity, depending on the microenvironment and the cytokine composition of the niche.41-43 A wide variety of macrophage subtypes has been described both in vitro and in vivo. The 2 extremes are represented by “classically activated” or M1 macrophages, which are induced by microbial agents and proinflammatory T-helper (Th)1 cytokines (interferon-γ, lipopolysaccharide), and “alternatively activated” or M2 macrophages, mainly induced by Th2 cytokines (IL-4, IL-10, IL-13).41-45 These macrophage populations are functionally different: M1 cells have inflammatory functions and bactericidal activity and produce high levels of proinflammatory cytokines and reactive nitrogen and oxygen radicals; M2 cells have immunoregulatory functions, aid parasite clearance, show increased phagocytic activity, and are involved in cell growth control, matrix remodeling, angiogenesis, and tissue repair.
Recent studies suggest that M1 and M2 macrophages show different expression profiles of iron-related genes.46-49 However, the question whether Hb, heme, or iron could directly affect the phenotype of macrophages and whether heme/iron scavengers could alter or revert this effect has never been investigated. Despite the fact that the dichotomous M1/M2 nomenclature often appears as an oversimplification and does not fully reflect on the complex biology of macrophage subsets, in this study, we took these phenotypes as a reference and analyzed expression of established M1 and M2 markers to define whether heme and iron polarize macrophages toward a pro- or anti-inflammatory phenotype.
We now show that heme, and specifically its iron moiety, promotes pronounced phenotypic changes in macrophages toward an M1-like proinflammatory phenotype, a response mediated by TLR4 signaling and reactive oxygen species (ROS) production. Importantly, the heme scavenger Hx prevents heme-induced proinflammatory phenotypic switching, both in vitro and in vivo. Additionally, administration of exogenous Hx in an experimental model for hemolysis and a mouse model of SCD is beneficial to counteract the heme-driven proinflammatory status of macrophages and its pathophysiologic consequences. These findings have important implication for the control of the inflammatory response in patients showing acute or chronic Hb and heme release.50
Materials and methods
Mouse models and treatments
Wild-type, Hx-null,51 TLR4-null52 (on B6J or SV129 genetic background), and knock-in HbA and HbS sickle mice (on mixed genetic background)53 were housed in the animal facility of the Molecular Biotechnology Center (Turin) or Innerbetriebliche Fortbildung (Heidelberg, only for bone marrow–derived macrophage [BMDM] preparation) and maintained on a standard diet containing 200 ppm iron (Teklad 2018S; Harlan). Mouse breeding and experiments were approved by and conducted in compliance with the guidelines of the University of Heidelberg (Germany) and Turin (Italy).
Cell treatment
Raw264.7 cells and BMDMs were treated with 2 × 107 hemolytic aged RBCs, 5 to 15 μM heme or Zn-mesoporphyrin bound to 5 to 15 μM albumin or Hx, or with 100 μM iron-nitrilotriacetate (FeNTA) alone or bound to 100 μM desferrioxamine (DFO).
Preparation of splenic and hepatic macrophages and flow cytometry analysis
Splenic and hepatic macrophage-enriched fractions, obtained by spleen mechanical disruption or liver enzymatic digestion, were analyzed by flow cytometry, as described in supplemental Data available on the Blood Web site.
Statistical analysis
Results were expressed as mean ± standard error of the mean (SEM). Comparisons between 2 groups were performed with 2-sided Welch t tests, and among 3 or >3 groups with 1- or 2-way analyses of variance, respectively, followed by the Bonferroni posttest. P < .05 was considered significant.
Further details on materials and methods have been included in supplemental Materials and Methods.
Results
Lack of hemopexin promotes heme loading of splenic myeloid cells
Recently, we demonstrated that Hx controls hepatic heme uptake and thus limits heme accumulation in extrahepatic tissues, such as the vessel wall and the heart,5 preventing heme-induced toxicity and tissue injury.5
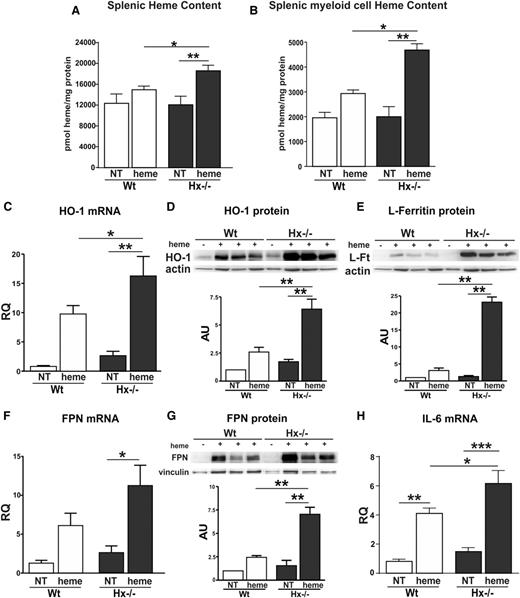
Here we show that heme-challenged Hx-deficient mice, a model for acute hemolytic event, accumulate more heme in the spleen compared with heme-treated wild-type controls (Figure 1A). Consistently, CD11b+cells (granulocytes and monocytes) and iron-rich cells (macrophages) isolated from heme-treated Hx-null mice show a higher heme content and increased mRNA and protein expression of HO-1, L-ferritin, and Fpn (Figure 1B-G) and elevated mRNA levels of the proinflammatory cytokine IL-6 (Figure 1H). These data suggest that Hx limits heme uptake by white cell populations and that heme accumulation affects the expression of genes involved in iron handling and the inflammatory response.
Hx protects macrophages from heme overload. (A-B) Heme content in the spleen and in splenic myeloid cells (CD11b+ granulocytes, monocytes, and ferromagnetic macrophages) from untreated (NT) and heme-treated (+heme) (hemin 70 μmol/kg; 1 hour after injection) wild-type (Wt) and Hx-null (Hx−/−) mice (n = 3). (C-G) HO-1, L-ferritin, and FPN mRNA and protein expression in splenic myeloid cells (granulocytes, monocytes, and macrophages) isolated from the spleen of untreated (NT) or heme-treated (hemin 70 μmol/kg; 6 hours after injection) wild-type and Hx-null mice. In C and F, HO-1 and FPN mRNA levels were analyzed by qRT-PCR (NT: n = 5; heme: n = 6). Representative western blots of HO-1, L-ferritin, and FPN are shown in D, E, and G (NT: n = 3; heme: n = 6). (H) qRT-PCR analysis of IL-6 mRNA expression in splenic myeloid cells isolated from the spleen of untreated (NT) or heme-treated wild-type and Hx-null mice (hemin 70 μmol/kg; 6 hours after injection) (untreated, NT: n = 3; heme: n = 6). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001. mRNA levels measured by qRT-PCR are expressed in relative quantity (RQ) to the untreated (NT) sample (RQ = 1); densitometric analysis is reported in arbitrary units (AUs), as the ratio to the untreated (NT) sample (AU = 1).
Hx protects macrophages from heme overload. (A-B) Heme content in the spleen and in splenic myeloid cells (CD11b+ granulocytes, monocytes, and ferromagnetic macrophages) from untreated (NT) and heme-treated (+heme) (hemin 70 μmol/kg; 1 hour after injection) wild-type (Wt) and Hx-null (Hx−/−) mice (n = 3). (C-G) HO-1, L-ferritin, and FPN mRNA and protein expression in splenic myeloid cells (granulocytes, monocytes, and macrophages) isolated from the spleen of untreated (NT) or heme-treated (hemin 70 μmol/kg; 6 hours after injection) wild-type and Hx-null mice. In C and F, HO-1 and FPN mRNA levels were analyzed by qRT-PCR (NT: n = 5; heme: n = 6). Representative western blots of HO-1, L-ferritin, and FPN are shown in D, E, and G (NT: n = 3; heme: n = 6). (H) qRT-PCR analysis of IL-6 mRNA expression in splenic myeloid cells isolated from the spleen of untreated (NT) or heme-treated wild-type and Hx-null mice (hemin 70 μmol/kg; 6 hours after injection) (untreated, NT: n = 3; heme: n = 6). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001. mRNA levels measured by qRT-PCR are expressed in relative quantity (RQ) to the untreated (NT) sample (RQ = 1); densitometric analysis is reported in arbitrary units (AUs), as the ratio to the untreated (NT) sample (AU = 1).
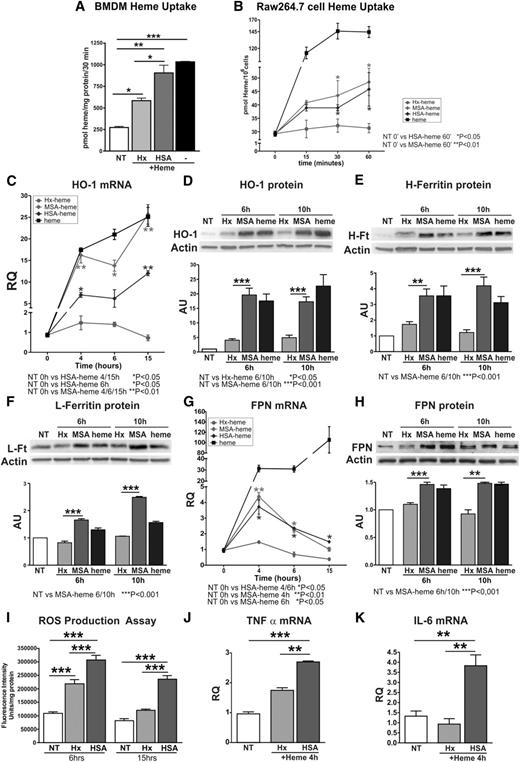
To directly demonstrate that Hx reduces heme accumulation specifically in macrophages, we measured heme levels in BMDMs incubated with 5 μM heme bound to albumin (human/murine serum albumin [HSA/MSA]) or Hx (human) in a 1:1 ratio. Consistent with our in vivo findings in Hx-deficient mice, heme levels were significantly increased in BMDMs treated with heme-albumin compared with those treated with heme-Hx (Figure 2A). Furthermore, monocyte/macrophage-like cells (Raw264.7) treated with heme-albumin accumulated higher levels of heme (Figure 2B). Similar results were obtained when cells were exposed to the fluorescent heme analog zinc-mesoporphyrin (ZnMP) bound to albumin or Hx (supplemental Figure 1). Raw 264.7 cells treated with heme-albumin showed increased mRNA and protein expression of HO-1 and FPN, increased protein expression of H- and L-ferritin, and HO activity compared with heme-Hx treated cells (Figure 2C-H; Table 1). In addition, we observed increased ROS production (Figure 2I; supplemental Figure 2), as well as an enhanced expression of IL-6 and TNFα in cells treated with heme-albumin compared with heme-Hx (Figure 2J-K).
Hx limits heme uptake, iron loading, and the production of ROS and cytokines in BMDM and Raw264.7 cells. (A) Heme levels in BMDMs treated with 5 μM Hx-heme or HSA-heme for 30 minutes (n = 3). (B) Heme levels in Raw264.7 cells treated with Hx-heme, HSA-heme, MSA-heme, or heme alone for 15, 30, or 60 minutes (n = 3). (C-H) HO-1, H- and L-ferritin, and FPN expression in Raw264.7 cells incubated with 5 μM Hx-heme, MSA-heme, or heme alone for the indicated times. In C and G, HO-1 and FPN mRNAs were analyzed by qRT-PCR (n = 4). Representative western blots are shown in D, E, F, and H (untreated, NT: n = 3; heme: n = 9). (I) Measurement of ROS levels in Raw264.7 cells treated with 10 μM Hx-heme, HSA-heme, or heme alone (6 and 15 hours; n = 4). (J-K) qRT-PCR analysis of TNFα and IL-6 mRNA expression in Raw264.7 cells treated with 5 μM Hx-heme or HSA-heme for 4 hours (n = 6). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001. mRNA levels measured by qRT-PCR are expressed in RQ to the untreated (NT) sample (RQ = 1); densitometric analysis is reported in AU, as the ratio to the untreated (NT) sample (AU = 1).
Hx limits heme uptake, iron loading, and the production of ROS and cytokines in BMDM and Raw264.7 cells. (A) Heme levels in BMDMs treated with 5 μM Hx-heme or HSA-heme for 30 minutes (n = 3). (B) Heme levels in Raw264.7 cells treated with Hx-heme, HSA-heme, MSA-heme, or heme alone for 15, 30, or 60 minutes (n = 3). (C-H) HO-1, H- and L-ferritin, and FPN expression in Raw264.7 cells incubated with 5 μM Hx-heme, MSA-heme, or heme alone for the indicated times. In C and G, HO-1 and FPN mRNAs were analyzed by qRT-PCR (n = 4). Representative western blots are shown in D, E, F, and H (untreated, NT: n = 3; heme: n = 9). (I) Measurement of ROS levels in Raw264.7 cells treated with 10 μM Hx-heme, HSA-heme, or heme alone (6 and 15 hours; n = 4). (J-K) qRT-PCR analysis of TNFα and IL-6 mRNA expression in Raw264.7 cells treated with 5 μM Hx-heme or HSA-heme for 4 hours (n = 6). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001. mRNA levels measured by qRT-PCR are expressed in RQ to the untreated (NT) sample (RQ = 1); densitometric analysis is reported in AU, as the ratio to the untreated (NT) sample (AU = 1).
Raw 264.7 macrophages heme-oxygenase activity assay
| Treatment . | pmol Bilirubin/mg microsomal protein/hour . |
|---|---|
| NT | 181.165 ± 20.091 |
| Hx-heme | 183.977 ± 10.261 |
| HSA-heme | 264.141 ± 5.582 vs NT; vs Hx-heme **P < .01 |
| MSA-heme | 246.633 ± 11.161 vs NT; vs Hx-heme *P < .05 |
| Heme | 376.744 ± 11.083 vs NT; vs Hx-heme *P < .05 |
| Treatment . | pmol Bilirubin/mg microsomal protein/hour . |
|---|---|
| NT | 181.165 ± 20.091 |
| Hx-heme | 183.977 ± 10.261 |
| HSA-heme | 264.141 ± 5.582 vs NT; vs Hx-heme **P < .01 |
| MSA-heme | 246.633 ± 11.161 vs NT; vs Hx-heme *P < .05 |
| Heme | 376.744 ± 11.083 vs NT; vs Hx-heme *P < .05 |
The assay was performed on microsomes purified from Raw 264.7 cells untreated (NT) or treated with 15 μM Hx-heme, HSA-heme, MSA-heme, or heme alone for 5 hours. Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001. n = 4 for each sample.
Taken together, these data demonstrate that Hx limits macrophage heme overload and prevents the pro-oxidant and proinflammatory effects triggered by heme in cellular assays and in vivo.
Heme and iron polarize macrophages toward an M1-like proinflammatory phenotype
Based on the observation that ROS production and expression of proinflammatory cytokines are elevated in heme-loaded macrophages and that heme triggers sterile inflammation,18,54 we hypothesized that heme may induce macrophage polarization toward an inflammatory phenotype.
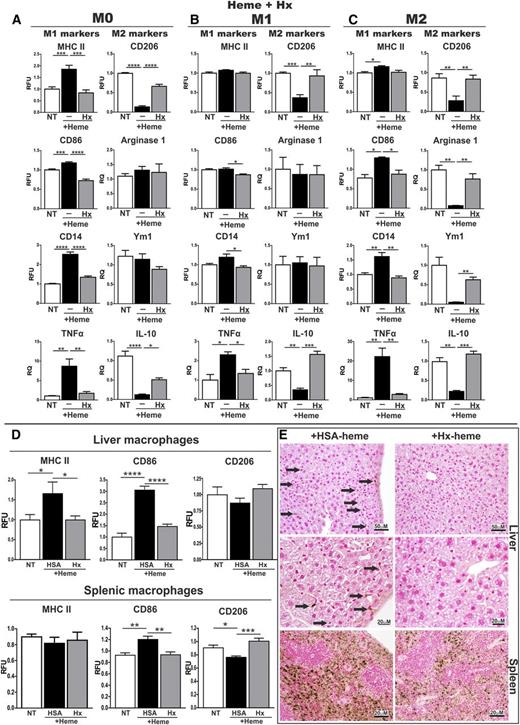
To test this, we exposed cultured BMDMs (M0: not treated with cytokines) to aged hemolytic RBCs (2 × 107 cells) as a source of Hb, to hemin (5 μM bound to bovine serum albumin [BSA], 1:1 ratio) or to FeNTA (100 μM) and analyzed the expression of M1 and M2 macrophage polarization markers by flow cytometry (gating strategy; supplemental Figure 3) and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). We show that incubation of M0 BMDMs with aged RBCs increased the expression of M1 polarization markers, in particular MHCII and TNFα, and decreased the expression of the M2 polarization marker CD206 (supplemental Figure 4). Likewise, heme and FeNTA treatment causes the induction of the M1 markers MHCII, CD86, CD14, TNFα, IL-6, and IL1β and a decrease in the M2 markers CD206, IL-10, and Arginase-1 (the last with FeNTA only) in M0 BMDMs (Figure 3A; supplemental Figures 5, 6A, and 7). We next investigated whether heme and iron could also affect the differentiation of macrophages that were exposed together with cytokines that trigger M1 or M2 polarization. We demonstrate that both hemin and FeNTA treatment enhanced the phenotype of M1 macrophages (Figure 3B; supplemental Figures 6B and 7) and shifted the differentiation of M2 macrophages toward an M1-like phenotype (Figure 3C; supplemental Figures 6C and 7). Importantly, protoporphyrin did not induce TNFα, suggesting a critical proinflammatory role for iron within the heme moiety (supplemental Figure 8). Regulation of iron-related genes in M0, M1, and M2 macrophages was monitored to control for the efficacy of heme and FeNTA treatment (supplemental Figure 9). These results demonstrate that heme induces polarization of macrophages toward a M1-like proinflammatory phenotype and that this effect is independent of the cell differentiation state.
Heme induces macrophage polarization toward an M1-like phenotype, independently of the cell differentiation state. (A) M0, (B) M1, and (C) M2 BMDMs were left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; 1:1 ratio) for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. The expression of major histocompatibility complex class II (MHCII), CD86, CD14, and CD206 was analyzed by flow cytometry and is expressed in relative fluorescence units (RFU) as fold change compared with untreated cells (NT). The mRNA levels of TNFα, Arginase-1, Ym1, and IL-10 were analyzed by qRT-PCR and expressed in RQ, as fold change compared with untreated cells (NT). Results shown are the average of ≥3 independent experiments. (A direct comparison of expression levels of M1 and M2 markers in M0, M1, and M2 BMDMs is shown in supplemental Figure 7, where fold changes are compared with untreated [NT] M0 cells). (D) Analysis of the M1 markers MHCII and CD86 and the M2 marker CD206 and Arginase-1 in macrophages isolated from liver or spleen of untreated (NT) or heme-treated (hemin 30 μmol/kg) wild-type mice. Macrophage were analyzed 15 hours or 15 days after intravenous heme injection (n = 6). (E) Hematoxylin/eosin and Picrosirius red staining for collagen on liver sections from heme-treated wild-type mice (70 μmol/kg, 5 injections; scale bar, 300-100 μm). Arrows indicate infiltrates (hem/eo) and collagen (picrosirius). (F) Analysis of the M1 markers MHCII, CD86, TNFα, and IL-6 and the M2 marker CD206 in hepatic macrophages of control HbA and sickle HbS mice (n = 4). The expression of polarization markers in hepatic and splenic macrophages was analyzed by flow cytometry and is expressed in RFU as fold change compared with untreated wild-type mice (NT) or HbA controls. (G) Perls’ staining for iron on liver sections from HbA and HbS mice (scale bar, 100 μm). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001. Regulation of iron-related genes in M0, M1, and M2 macrophages was monitored to control for the efficacy of heme and FeNTA treatment (supplemental Figure 9).
Heme induces macrophage polarization toward an M1-like phenotype, independently of the cell differentiation state. (A) M0, (B) M1, and (C) M2 BMDMs were left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; 1:1 ratio) for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. The expression of major histocompatibility complex class II (MHCII), CD86, CD14, and CD206 was analyzed by flow cytometry and is expressed in relative fluorescence units (RFU) as fold change compared with untreated cells (NT). The mRNA levels of TNFα, Arginase-1, Ym1, and IL-10 were analyzed by qRT-PCR and expressed in RQ, as fold change compared with untreated cells (NT). Results shown are the average of ≥3 independent experiments. (A direct comparison of expression levels of M1 and M2 markers in M0, M1, and M2 BMDMs is shown in supplemental Figure 7, where fold changes are compared with untreated [NT] M0 cells). (D) Analysis of the M1 markers MHCII and CD86 and the M2 marker CD206 and Arginase-1 in macrophages isolated from liver or spleen of untreated (NT) or heme-treated (hemin 30 μmol/kg) wild-type mice. Macrophage were analyzed 15 hours or 15 days after intravenous heme injection (n = 6). (E) Hematoxylin/eosin and Picrosirius red staining for collagen on liver sections from heme-treated wild-type mice (70 μmol/kg, 5 injections; scale bar, 300-100 μm). Arrows indicate infiltrates (hem/eo) and collagen (picrosirius). (F) Analysis of the M1 markers MHCII, CD86, TNFα, and IL-6 and the M2 marker CD206 in hepatic macrophages of control HbA and sickle HbS mice (n = 4). The expression of polarization markers in hepatic and splenic macrophages was analyzed by flow cytometry and is expressed in RFU as fold change compared with untreated wild-type mice (NT) or HbA controls. (G) Perls’ staining for iron on liver sections from HbA and HbS mice (scale bar, 100 μm). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001. Regulation of iron-related genes in M0, M1, and M2 macrophages was monitored to control for the efficacy of heme and FeNTA treatment (supplemental Figure 9).
To assess whether heme alters macrophage plasticity in vivo, we injected wild-type mice intravenously with heme (30 μmol/kg) and analyzed iron levels and the expression of polarization markers by flow cytometry in spleen and liver macrophages, 15 hours or 15 days after heme injection (gating strategy; supplemental Figures 10 and 11). Importantly, 15 hours after heme injection, hepatic macrophages showed iron deposition (data not shown) and an increased expression of the M1 markers MHCII and CD86 and reduced levels of the M2 markers CD206 and Arginase-1 compared with nontreated mice. Likewise, splenic macrophages from heme-treated mice show increased expression of CD86 and reduced levels of CD206 and Arginase-1 (Figure 3D). Modulation of most M1 and M2 markers was reduced 15 days after heme injection, indicating that heme-induced proinflammatory activation of macrophages is a reversible event.
The polarization of macrophages into an M1 proinflammatory phenotype contributes to the perpetuation of inflammation by recruitment of circulating monocytes and leads to hepatocyte apoptosis and fibrosis.55 Consistently, we show that repeated heme injections (70 μmol/kg, 5 injections) cause the recruitment of a massive number of inflammatory cells, deposition of collagen fiber, and liver damage (Figure 3E).
We next assessed whether our findings are of pathophysiologic relevance for a paradigmatic chronic hemolytic disorder such as SCD that is hallmarked by a high amount of circulating Hb/heme, accelerated erythrophagocytosis, and low endogenous levels of Hp and Hx.5 Our analysis shows that liver macrophages from HbS sickle mice express significant higher levels of the M1 markers MHCII, CD86, TNFα, and IL-6 compared with HbA control mice and have enhanced macrophage iron deposition (Figure 3F-G). Taken together, our data demonstrate that heme triggers reversible proinflammatory phenotypic switching of macrophages in vivo that could partially contribute to the inflammatory state associated with chronic hemolytic conditions, such as SCD.
Activation of the TLR4 signaling pathway and ROS production control heme-mediated phenotypic changes of macrophages
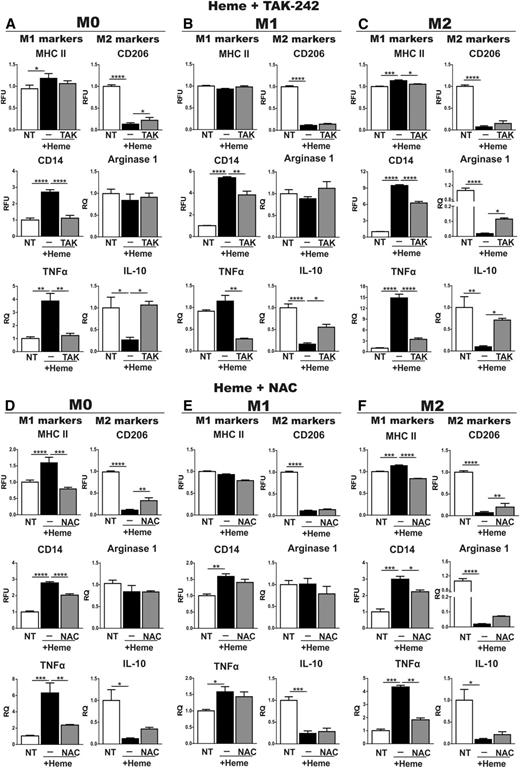
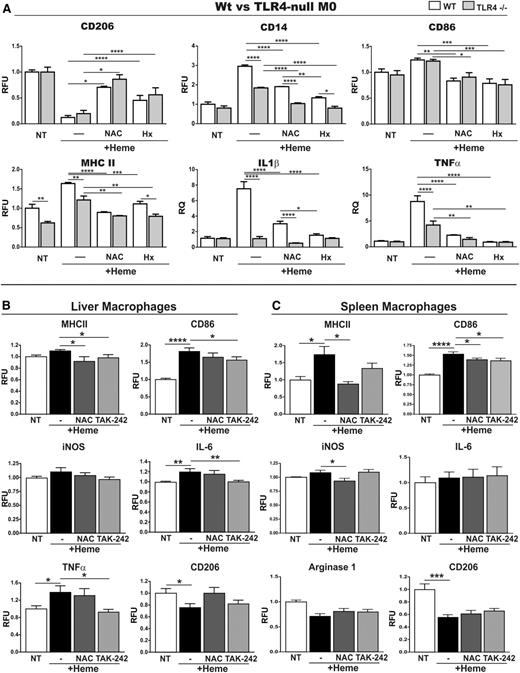
To study whether TLR4 activation and/or ROS production are involved in heme-induced M1 polarization of macrophages, BMDMs were treated either with heme alone or together with the TLR4 inhibitor TAK-242 (400 nM) or the antioxidant N-acetylcysteine (NAC, 2 mM). Cotreatment of M0 BMDMs with heme and TAK-242 attenuated the increase of the M1 markers MHCII, CD14, TNFα, IL-6, IL-1β, and CD86 and the decrease of the M2 marker CD206, IL-10, and Ym1 in comparison with heme treatment alone (Figure 4A; supplemental Figures 5 and 12). Similarly, NAC prevented increased expression of MHCII, CD14, TNFα, IL-6, IL-1β, and CD86 and mildly compensated for the decrease of CD206 and IL-10 following heme treatment (Figure 4D; supplemental Figure 5). In addition, both TAK-242 and NAC attenuated heme-mediated M1 marker induction and M2 marker decrease in M2 BMDMs, whereas a milder effect was observed in M1 BMDMs (Figure 4B-F). Consistent with the data obtained by applying the TLR4 inhibitor, BMDMs from TLR4-null mice show a strongly attenuated increase of the M1 markers CD14, MHCII, IL-1β, and TNFα on heme treatment. Additional NAC treatment of TLR4-null BMDMs fully rescued the residual heme-mediated induction of these markers (Figure 5A). Likewise, NAC was able to prevent iron-induced M1 polarization in M0, M1, and M2 BMDMs (supplemental Figure 13), indicating that the alteration of macrophage plasticity in response to iron is mostly explained by its pro-oxidant properties.
The TLR4 inhibitor TAK-242 and the antioxidant NAC attenuate heme-induced M1 polarization of macrophages. (A,D) M0, (B,E) M1, and (C,F) M2 BMDMs were left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; heme-BSA 1:1 ratio) with or without (A-C) 400 nM TAK-242 and (D-F) 2 mM NAC for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. The expression of MHCII, CD14, and CD206 was analyzed by flow cytometry and expressed in RFU as fold change to nontreated (NT) cells. mRNA levels of TNFα, Arginase-1, and IL-10 were analyzed by qRT-PCR and expressed in RQ, as fold change to untreated (NT) cells. Results shown are representative of 3 independent experiments. Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
The TLR4 inhibitor TAK-242 and the antioxidant NAC attenuate heme-induced M1 polarization of macrophages. (A,D) M0, (B,E) M1, and (C,F) M2 BMDMs were left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; heme-BSA 1:1 ratio) with or without (A-C) 400 nM TAK-242 and (D-F) 2 mM NAC for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. The expression of MHCII, CD14, and CD206 was analyzed by flow cytometry and expressed in RFU as fold change to nontreated (NT) cells. mRNA levels of TNFα, Arginase-1, and IL-10 were analyzed by qRT-PCR and expressed in RQ, as fold change to untreated (NT) cells. Results shown are representative of 3 independent experiments. Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
TLR4 signaling and ROS production contribute to heme-induced M1 proinflammatory phenotypic switching of macrophages. (A) M0 BMDMs were prepared from wild-type and TLR4-null mice and left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; heme-BSA 1:1 ratio) with or without 2 mM NAC or to 5 μM heme-Hx (Hx; heme-Hx 1:1 ratio) for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. The expression of CD206, CD14, CD86, and MHCII was analyzed by flow cytometry and expressed in RFU as fold change to untreated cells (NT). mRNA levels of TNFα and IL-1β were analyzed by qRT-PCR and expressed in RQ, as fold change to untreated cells (NT). Results shown are representative of 3 independent experiments. (B-C) Analysis of the M1 markers MHCII, CD86, iNOS, IL-6, and TNFα and the M2 markers Arginase-1 and CD206 in macrophages isolated from (B) liver or (C) spleen of wild-type mice untreated (NT) or treated with heme (heme-BSA 30 μmol/kg; 15 hours) with or without TAK-242 (2 mg/kg) or NAC (500 mg/kg) (n = 8). The expression of polarization markers in hepatic and splenic macrophages was analyzed by flow cytometry and is expressed in RFU as fold change compared with untreated wild-type mice (NT). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
TLR4 signaling and ROS production contribute to heme-induced M1 proinflammatory phenotypic switching of macrophages. (A) M0 BMDMs were prepared from wild-type and TLR4-null mice and left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; heme-BSA 1:1 ratio) with or without 2 mM NAC or to 5 μM heme-Hx (Hx; heme-Hx 1:1 ratio) for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. The expression of CD206, CD14, CD86, and MHCII was analyzed by flow cytometry and expressed in RFU as fold change to untreated cells (NT). mRNA levels of TNFα and IL-1β were analyzed by qRT-PCR and expressed in RQ, as fold change to untreated cells (NT). Results shown are representative of 3 independent experiments. (B-C) Analysis of the M1 markers MHCII, CD86, iNOS, IL-6, and TNFα and the M2 markers Arginase-1 and CD206 in macrophages isolated from (B) liver or (C) spleen of wild-type mice untreated (NT) or treated with heme (heme-BSA 30 μmol/kg; 15 hours) with or without TAK-242 (2 mg/kg) or NAC (500 mg/kg) (n = 8). The expression of polarization markers in hepatic and splenic macrophages was analyzed by flow cytometry and is expressed in RFU as fold change compared with untreated wild-type mice (NT). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
To test whether ROS production and/or TLR4 activation are crucial for the in vivo response of macrophages to heme, we challenged wild-type mice either with heme alone or together with NAC (500 mg/kg body weight) or TAK-242 (2 mg/kg body weight) and analyzed the expression of macrophage polarization markers in isolated macrophages. In hepatic and splenic macrophages, cotreatment with NAC or TAK-242 reduced the heme-induced upregulation of the M1 markers MHCII and CD86 and attenuated inducible nitric oxide synthase (iNOS) expression. In hepatic macrophages, TAK-242 also diminished heme-driven induction of IL-6 and TNFα and NAC partially recovered CD206 downregulation (Figure 5B-C). The partial rescue observed with both TAK-242 and NAC could be due to the use of suboptimal doses of drugs applied or additional pathways contributing to heme/iron-induced phenotypic switching of macrophages.
These findings suggest that both TLR4 activation and ROS production control heme-mediated programming of macrophages toward the M1 proinflammatory phenotype and that each of the 2 pathways is indicated by a partially overlapping subset of M1 and M2 markers.
The heme scavenger Hx and the iron chelator DFO prevent heme and iron-induced M1 polarization of macrophages
We next tested whether heme and iron scavengers prevent heme and iron-induced macrophage polarization, respectively. M0 BMDMs were exposed to hemolytic aged RBCs, heme, or FeNTA alone or together with Hx (100 μM with RBCs; 5 μM with heme) or DFO (100 μM with FeNTA). Treatment with Hx or DFO diminished the increase of some M1 polarization markers in BMDMs following treatment with hemolytic RBCs (supplemental Figure 14). In addition, Hx and DFO prevented the heme- and iron-mediated induction of the M1 markers MHCII, CD86, CD14, and TNFα and the decrease of some M2 markers, such as CD206, IL-10, and Arginase-1 (the last for FeNTA only) in M0 BMDMs (Figure 6A; supplemental Figure 15A). Importantly, the expression of polarization markers remained unaltered following treatment with Hx and DFO alone (supplemental Figure 16). Similar data were obtained when M1 or M2 macrophages were exposed to heme or FeNTA in the presence of Hx or DFO (Figure 6B-C; supplemental Figure 15B-C). Interestingly, treatment with Hx and DFO improved the survival of BMDMs exposed to heme and iron, respectively (Table 2).
Hx prevents M1 polarization of macrophages in response to heme. (A) M0, (B) M1, and (C) M2 BMDMs were left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; heme-BSA 1:1 ratio) or 5 μM heme-Hx (Hx; heme-Hx 1:1 ratio) for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. Results shown are the average of ≥3 independent experiments. (D) Analysis of the M1 markers MHCII and CD86 and the M2 marker CD206 in macrophages from liver and spleen of wild-type mice that remained untreated or were treated with heme-HSA or heme-Hx (complex 15 μmol/kg; 15 hours) (n = 5). The expression of MHCII, CD86, CD14, and CD206 was analyzed by flow cytometry and expressed in RFU as fold change to untreated cells or untreated wild-type animals (NT). mRNA levels of TNFα, Arginase-1, Ym1, and IL-10 were analyzed by qRT-PCR and expressed in RQ, as fold change to untreated cells (NT). (E) Perls’ staining for iron on liver and spleen sections from wild-type mice that were treated with heme-HSA or heme-Hx (scale bar, 20-50 μm). Arrows indicate iron-loaded Kupffer cells. Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Hx prevents M1 polarization of macrophages in response to heme. (A) M0, (B) M1, and (C) M2 BMDMs were left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; heme-BSA 1:1 ratio) or 5 μM heme-Hx (Hx; heme-Hx 1:1 ratio) for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. Results shown are the average of ≥3 independent experiments. (D) Analysis of the M1 markers MHCII and CD86 and the M2 marker CD206 in macrophages from liver and spleen of wild-type mice that remained untreated or were treated with heme-HSA or heme-Hx (complex 15 μmol/kg; 15 hours) (n = 5). The expression of MHCII, CD86, CD14, and CD206 was analyzed by flow cytometry and expressed in RFU as fold change to untreated cells or untreated wild-type animals (NT). mRNA levels of TNFα, Arginase-1, Ym1, and IL-10 were analyzed by qRT-PCR and expressed in RQ, as fold change to untreated cells (NT). (E) Perls’ staining for iron on liver and spleen sections from wild-type mice that were treated with heme-HSA or heme-Hx (scale bar, 20-50 μm). Arrows indicate iron-loaded Kupffer cells. Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Viability of BMDMs exposed to heme, FeNTA, and hemolytic RBCs in the presence or absence of Hx and DFO
| . | NT . | +Heme-BSA . | + Heme-Hx . |
|---|---|---|---|
| M0 | 89.07 ± 2.443 | 51.13 ± 5.032 vs NT P < .0001 | 86.25 ± 3.036 vs Heme-BSA P < .0001 |
| M1 | 63.88 ± 3.785 | 46.17 ± 2.569 vs NT P < .001 | 71.95 ± 2.737 vs Heme-BSA P < .0001 |
| M2 | 89.85 ± 1.699 | 52.08 ± 2.788 vs NT P < .0001 | 90.78 ± 0.895 vs Heme-BSA P < 00001 |
| +FeNTA | + FeNTA-DFO | ||
| M0 | 94.40 ± 0.503 | 72.03 ± 5.341 vs NT P < .05 | 90.23 ± 4.670 vs FeNTA P < .05 |
| M1 | 61.30 ± 6.584 | 45.07 ± 5.007 vs NT not significant | 55.65 ± 6.986 vs FeNTA not significant |
| M2 | 93.40 ± 1.320 | 73.13 ± 3.464 vs NT P < .01 | 88.80 ± 2.70 vs FeNTA P < .05 |
| +RBC | + RBC-Hx | ||
| M0 | 93.98 ± 0.349 | 83.02 ± 0.837 vs NT P < .0001 | 88.75 ± 0.150 vs RBC P < .001 |
| . | NT . | +Heme-BSA . | + Heme-Hx . |
|---|---|---|---|
| M0 | 89.07 ± 2.443 | 51.13 ± 5.032 vs NT P < .0001 | 86.25 ± 3.036 vs Heme-BSA P < .0001 |
| M1 | 63.88 ± 3.785 | 46.17 ± 2.569 vs NT P < .001 | 71.95 ± 2.737 vs Heme-BSA P < .0001 |
| M2 | 89.85 ± 1.699 | 52.08 ± 2.788 vs NT P < .0001 | 90.78 ± 0.895 vs Heme-BSA P < 00001 |
| +FeNTA | + FeNTA-DFO | ||
| M0 | 94.40 ± 0.503 | 72.03 ± 5.341 vs NT P < .05 | 90.23 ± 4.670 vs FeNTA P < .05 |
| M1 | 61.30 ± 6.584 | 45.07 ± 5.007 vs NT not significant | 55.65 ± 6.986 vs FeNTA not significant |
| M2 | 93.40 ± 1.320 | 73.13 ± 3.464 vs NT P < .01 | 88.80 ± 2.70 vs FeNTA P < .05 |
| +RBC | + RBC-Hx | ||
| M0 | 93.98 ± 0.349 | 83.02 ± 0.837 vs NT P < .0001 | 88.75 ± 0.150 vs RBC P < .001 |
Cell viability was assessed by flow cytometry using 7AAD. Viability is reported as percentage viable cells over the total number of analyzed cells.
To assess the potential of heme scavengers for protecting macrophages from heme-triggered polarization in vivo, we injected wild-type mice with heme bound to the low-affinity scavenger, HSA, or the high affinity scavenger, Hx, and analyzed macrophage polarization markers. Interestingly, hepatic macrophages derived from mice treated with heme-Hx showed a blunted induction of the M1 markers MHCII and CD86 compared with cells from animals treated with heme-HSA (complex 15 μmol/kg; 15 hours). Similarly, the heme-mediated induction of the M1 marker CD86 and downregulation of the M2 marker CD206 are rescued in splenic macrophages from heme-Hx-treated mice compared with heme-HSA–challenged animals (Figure 6D). Perls’ staining of liver and spleen sections demonstrates that macrophages accumulated more iron following heme-HSA treatment compared with heme-Hx treatment (Figure 6E).
These results suggest that the sequestration of heme and iron by specific scavengers counteracts the proinflammatory state of macrophages associated with M1 differentiation.
Hemopexin therapy ameliorates the proinflammatory status of macrophages in a mouse model of sickle cell disease
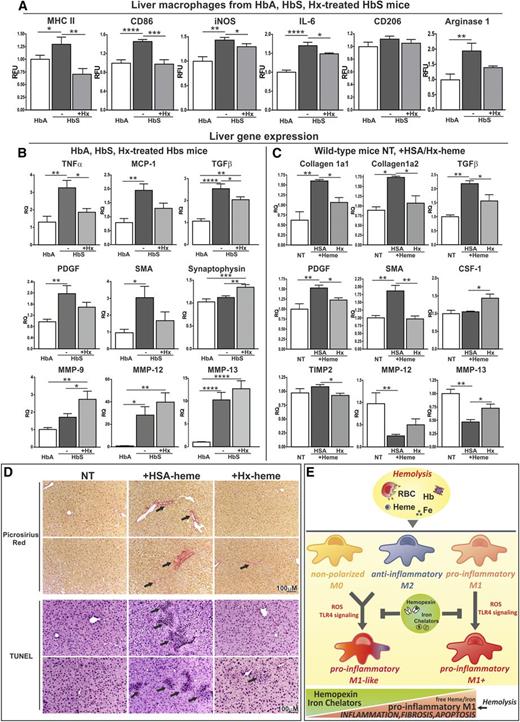
We next evaluated whether Hx administration is able to attenuate the proinflammatory status of macrophages in a murine disease model for SCD. The treatment of sickle mice with human Hx (4 mg Hx intraperitoneally once a week, for 3 weeks) decreased the expression of the M1 markers MHCII, CD86, iNOS, and IL-6 in hepatic macrophages compared with HbA control mice, whereas the M2 marker CD206 was not significantly altered. Higher expression of Arginase-1 may be related to the liver fibrotic damage that SCD mice tend to develop, as Arginase-1 contributes to collagen synthesis. Interestingly, the attenuated levels of Arginase-1 following Hx injection may be a consequence of reduced heme-mediated oxidative tissue damage and fibrosis (Figure 7A).
Hx rescues heme-induced M1 polarization of macrophages in a mouse model of sickle cell disease. (A) Analysis of the M1 markers MHCII, CD86, iNOS, and IL-6 and the M2 markers CD206 and Arginase-1 in macrophages from liver of HbA mice and HbS mice that remained untreated or were treated with Hx (4 mg intraperitoneally once a week for 3 weeks) (n = 5). The expression of polarization markers was analyzed by flow cytometry and is expressed in RFU as fold change to HbA animals. (B) qRT-PCR analysis of hepatic mRNA levels of TNFα, MCP-1, TGFβ, PDGF, MMP-9, MMP-12, MMP-13, synaptophysin, and SMA in HbA mice and HbS mice that remained untreated or were treated with Hx (4 mg intraperitoneally once a week for 3 weeks) (n = 7). (C) qRT-PCR analysis of hepatic mRNA levels of collagen1a1, collagen1a2, TGFβ, PDGF, SMA, colony stimulating factor-1, TIMP2, MMP-12, and MMP-13 in wild-type mice that remained untreated or were treated with heme-HSA or heme-Hx (complex 5 μmol/kg, 7 injections over 2 weeks) (n = 5). (D) Picrosirius red staining for collagen and Tunel staining for apoptosis on liver sections from wild-type mice that remained untreated or were treated with heme-HSA or heme-Hx (complex 5 μmol/kg, 7 injections over 2 weeks) (scale bar, 300-100 μm). Arrows indicate collagen (picrosirius) and apoptotic cells (tunel). Results shown are representative of 3 independent experiments. Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001. (E) Model for macrophage differentiation in response to Hb, heme, and iron and its protection by heme and iron scavengers. Hb derived from hemolytic RBCs, heme, and iron promotes macrophage differentiation toward an M1-like proinflammatory phenotype, via ROS production and TLR4 signaling activation. This effect is independent of the differentiation state of the macrophages (eg, M0, M1, or M2). Interestingly, M1 macrophages show a potentiated M1 phenotype (M1+), and even anti-inflammatory M2 macrophages can be shifted to an M1-like proinflammatory phenotype in response to heme and iron, suggesting that these signals dominate over those triggered by cytokines. Heme or iron scavengers, such as hemopexin (Hx) or desferrioxamine (DFO), protect macrophages from heme or iron-induced M1 polarization, reducing iron loading, cytokine production, and ROS formation.
Hx rescues heme-induced M1 polarization of macrophages in a mouse model of sickle cell disease. (A) Analysis of the M1 markers MHCII, CD86, iNOS, and IL-6 and the M2 markers CD206 and Arginase-1 in macrophages from liver of HbA mice and HbS mice that remained untreated or were treated with Hx (4 mg intraperitoneally once a week for 3 weeks) (n = 5). The expression of polarization markers was analyzed by flow cytometry and is expressed in RFU as fold change to HbA animals. (B) qRT-PCR analysis of hepatic mRNA levels of TNFα, MCP-1, TGFβ, PDGF, MMP-9, MMP-12, MMP-13, synaptophysin, and SMA in HbA mice and HbS mice that remained untreated or were treated with Hx (4 mg intraperitoneally once a week for 3 weeks) (n = 7). (C) qRT-PCR analysis of hepatic mRNA levels of collagen1a1, collagen1a2, TGFβ, PDGF, SMA, colony stimulating factor-1, TIMP2, MMP-12, and MMP-13 in wild-type mice that remained untreated or were treated with heme-HSA or heme-Hx (complex 5 μmol/kg, 7 injections over 2 weeks) (n = 5). (D) Picrosirius red staining for collagen and Tunel staining for apoptosis on liver sections from wild-type mice that remained untreated or were treated with heme-HSA or heme-Hx (complex 5 μmol/kg, 7 injections over 2 weeks) (scale bar, 300-100 μm). Arrows indicate collagen (picrosirius) and apoptotic cells (tunel). Results shown are representative of 3 independent experiments. Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001. (E) Model for macrophage differentiation in response to Hb, heme, and iron and its protection by heme and iron scavengers. Hb derived from hemolytic RBCs, heme, and iron promotes macrophage differentiation toward an M1-like proinflammatory phenotype, via ROS production and TLR4 signaling activation. This effect is independent of the differentiation state of the macrophages (eg, M0, M1, or M2). Interestingly, M1 macrophages show a potentiated M1 phenotype (M1+), and even anti-inflammatory M2 macrophages can be shifted to an M1-like proinflammatory phenotype in response to heme and iron, suggesting that these signals dominate over those triggered by cytokines. Heme or iron scavengers, such as hemopexin (Hx) or desferrioxamine (DFO), protect macrophages from heme or iron-induced M1 polarization, reducing iron loading, cytokine production, and ROS formation.
Proinflammatory activation of hepatic resident macrophages is known to induce blood monocyte recruitment, which enhances cytokine production. These cytokines cause hepatocyte apoptosis and promote fibrosis by inducing activation and transdifferentiation of hepatic stellate cells (HSCs) into myofibroblasts, which is the major collagen-producing cell type. Here we show that Hx treatment decreases expression of the M1 cytokine TNFα, the monocyte chemoattractant protein (MCP)1, and the profibrotic and mitogenic cytokines transforming growth factor (TGF)β and platelet-derived growth factor (PDGF) in the liver of sickle mice. Moreover, Hx administration increases hepatic expression of the matrix metalloproteinases MMP-9, MMP-12, and MMP-13 (mostly expressed by M2 macrophages). It further increases the expression of a marker for resting HSCs, synaptophysin, and reduces the expression of a marker for activated myofibroblast-like HSCs, smooth muscle actin (SMA) (Figure 7B).
Despite these favorable alterations observed in Hx-treated sickle mice, liver damage as measured by collagen deposition and apoptosis of hepatocytes could not be reverted (supplemental Figure 17). In 2- to 3-month-old sickle mice, liver damage is already established, and thus the right timing and dosage of Hx administration may need to be optimized.
We therefore next analyzed heme-Hx-treated wild-type mice that showed decreased hepatic expression of collagens, TGFβ, PDGF, SMA, and the tissue inhibitor of MMPs, TIMP2, as well as increased expression of the M2 polarizing colony stimulating factor-1 and MMPs compared with heme-HSA–treated mice (Figure 7C). In addition, wild-type mice treated with heme-Hx showed reduced liver collagen deposition and hepatocyte apoptosis compared with animals treated with heme-HSA (complex 5 μmol/kg; 7 injections over 2 weeks) (Figure 7D). Consistently, heme-treated Hx-null mice show increased inflammatory cell recruitment and tissue damage in the liver compared with heme-treated wild-type mice (70 μmol heme/kg; 5 intravenous injections) (supplemental Figure 18).
Taken together, these data suggest that Hx administration decreases heme-driven proinflammatory activation of macrophages. As a consequence, the release of inflammatory cytokines that drive monocyte recruitment and HSC transdifferentiation is reduced, and expression of MMPs that favors scar regression is increased. This results in the resolution of inflammation and decreased hepatic collagen deposition and cell apoptosis. In conclusion, an Hx-based therapy shows beneficial effects, in that it counteracts heme-induced proinflammatory activation of macrophages and attenuates some of its pathophysiologic consequences, such as chronic inflammation, hepatic fibrosis, and apoptosis (Figure 7E).
Discussion
In this study, we show that various sources of heme (hemolytic RBCs, free heme) and iron activate macrophages to differentiate toward an M1-like proinflammatory phenotype, which is hallmarked by the production of inflammatory cytokines and ROS. The underlying mechanisms involve ROS production and TLR4-controlled signaling. We further demonstrate that heme/iron-controlled macrophage polarization is preventable by heme scavengers or iron chelators. These findings are of relevance for diseases that are characterized by excessive circulating Hb, heme, and/or iron levels and macrophage iron loading.
Macrophages show high plasticity in response to the cytokine composition of the microenvironment. Our data show that Hb, heme, or iron in the microenvironment additionally affect macrophage plasticity. This finding extends previous work that demonstrates that cytokines that drive M1 or M2 polarization determine iron handling.46,47,56 In those studies, M1 macrophages show an iron retention phenotype and a gene expression profile with low expression of HO-1, FPN, and CD163 and high expression of ferritin. On the other hand, M2 macrophages show higher expression of HO-1, FPN, and CD163 and low expression of ferritin, likely associated with an iron release phenotype with increased iron uptake, recycling, and export activity. Our data are not entirely consistent with these observations, in that transferrin receptor 1 mRNA levels are higher in M2 compared with M1 macrophages,46,47 as reported,44,45 whereas HO-1 and FPN mRNA levels do not differ (supplemental Figure 9). These differences may be explained by the protocols applied for monocyte/macrophage differentiation. Despite differences in gene expression patterns, our findings, together with those by Recalcati et al46 and Corna et al,47 strengthen the concept that macrophage function and iron metabolism are tightly interconnected. Our study further demonstrates that the functional consequences of macrophage exposure to cytokines and iron integrate and eventually potentiate each other, according to their relative amounts available in the microenvironment, rather than having a mutually exclusive effect on macrophage polarization. It is important to note that heme and iron seem to exert a dominant effect on macrophage polarization, inducing a shift toward a proinflammatory phenotype. In particular, the polarization program of macrophages in response to cytokines can be enhanced (M1→M1+) or even shifted (M2→M1) by an additional exposure to a heme or an iron source (Figure 7D). Therefore, heme/iron accumulation is expected to modulate the phenotypical differences that macrophages acquire in response to the environmental stimuli.
Recently, a key role for heme in controlling monocyte differentiation into macrophages was demonstrated. This process is increased during pathologic hemolysis.57 Here we demonstrate that heme additionally affects the phenotype of differentiated macrophages. An involvement of Hb, heme, and iron in macrophage polarization was postulated in recent studies. In models of chronic venous leg ulcers and wound healing, macrophage iron overload correlates with a proinflammatory M1 phenotype, TNFα, and hydroxyl radical production.58 Nieuwenhuizen et al59 observed a decrease in M2 and an increase in M1 macrophages in the red pulp of the spleen and in synovial tissue in hemophilic mice. The authors speculated that this results from an increased number of damaged RBCs and a higher level of circulating Hb following hemarthrosis. The data presented here may explain these findings. Macrophage polarization has also been analyzed in atherosclerotic plaques, where erythrophagocytosis in hemorrhagic areas represents an important source of iron for macrophages.60-64 Hemorrhage-associated macrophages (HA or M-hem) in atherosclerotic lesions express high levels of the Hb-Hp scavenger receptor CD163, HO-1, and FPN, accounting for an increased capacity to handle Hb, facilitated heme catabolism and reduced intracellular free iron. Therefore, in atheroma, the Hb-Hp complex might model macrophages toward a protective HA-macrophage subtype with increased capacity to handle Hb, as well as antioxidative and antiatherogenic properties. In this regard, the saturation of the Hp system is expected to be detrimental, leading to uncontrolled Hb uptake and macrophage activation.
Consistently, we show that the saturation of the Hx system leads to heme accumulation and proinflammatory activation of macrophages. Heme released from cell-free Hb is normally bound to Hx and internalized by hepatocytes, via the LRP1 receptor,65 or other thus far unidentified and more specific scavengers. Hx-mediated heme scavenging and hepatic detoxification ensure protection of several extrahepatic tissues against heme toxicity.5,6 Here we show that this protective effect extends toward macrophages and is reduced in disorders where the Hx binding capacity is overwhelmed and its synthesis is not appropriate compared with the rate of consumption.5,15 This is the case in a mouse model of SCD, which is hallmarked by hemolysis, increased circulating Hb/heme, and low levels of Hp and Hx and shows elevated hepatic macrophage iron levels and M1 polarization.
In our study we further provide mechanistic insight into how heme and iron cause phenotypic changes of macrophages. Here we show that this process depends on the production of ROS and the activation of the TLR4 signaling pathway. This is strengthened by the observation that heme-mediated induction of M1 markers is attenuated in TLR4-null BMDMs and fully abolished when these cells are additionally exposed to NAC. Importantly, by scavenging free heme, Hx prevents heme-induced M1 macrophage polarization and thus avoids both TLR4 activation and ROS formation. These observations are in line with recent studies demonstrating antioxidant and anti-inflammatory properties of Hx,5,6,66-68 as well as its ability to prevent heme-triggered TLR4 activation and TNFα production.17,54 Thus, Hx treatment provides a clear advantage over pharmacologic approaches in that it combines antioxidant properties together with inhibitory effects on the proinflammatory pathways induced by heme.
Our findings are therefore expected to be relevant for those pathologies in which Hb and heme are released from the RBCs, systemically or locally, and Hp/Hx levels are reduced, thus leading to the activation of innate immune cells and the production of ROS and inflammatory cytokines. We suggest that the extent of inflammation associated with hemolytic disorders may depend on intravascular rather than extravascular hemolysis. In SCD, hemolysis occurs both intra- and extravascular and macrophage exposure to “extracellular” heme triggers inflammation via TLR4 stimulation. In other conditions, such as hereditary spherocytosis, hemolysis is mainly extravascular and RBCs are presented intact to reticuloendothelial system macrophages, thus explaining the milder signs of inflammation in these patients. The extent of inflammation in different hemolytic disorders may therefore depend on how macrophages get in contact with heme, either with heme contained within intact RBCs or in the form of free or bound Hb/heme. Controlled iron accumulation in macrophages, as a consequence of erythrophagocytosis or uptake of iron-bound transferrin, is not expected to represent a stimulus to trigger M1 polarization in vivo.
We propose that the modulation of macrophage polarization toward a less inflammatory phenotype may represent an interesting prophylactic or therapeutic approach in hemolytic disorders. This could be achieved by administration of heme and/or iron scavengers. Recent studies in mouse models of hemolytic disorders demonstrated that the therapeutic administration of Hx increases hepatic heme recovery and detoxification and successfully counteracts heme-induced endothelial activation and dysfunction.5 Similarly, in a SCD mouse model, vascular stasis and respiratory failure due to acute chest syndrome were prevented by treatment with purified or recombinant Hx.17,69 Here we show that the administration of Hx attenuates the induction of inflammatory cytokines and M1 polarization markers in macrophages triggered by heme. The activation of macrophages toward a proinflammatory phenotype in the liver contributes to the induction of hepatocyte apoptosis, perpetuation of inflammation via leukocyte recruitment, and promotion of fibrosis via cytokine release and HSC activation.55 Previously, we showed that Hx therapy attenuates hepatic lipid peroxidation, leukocyte recruitment, and the expression of inflammatory cytokines, such as IL-6 and TNFα, in SCD.5,6 Here we additionally show that Hx administration reduces the expression of the chomattractant MCP-1, the profibrotic TGFβ and PDGF, and the activation of HSCs in sickle mice, suggesting that Hx-mediated heme scavenging counteracts heme-driven hepatic fibrosis and tissue injury.
Our data support the idea that heme-induced phenotypic switching of macrophages toward a proinflammatory phenotype can contribute to the exacerbation of inflammation and chronic tissue injury in hemolytic disorders and that Hx therapy could alleviate these pathophysiologic consequences by preventing macrophage inflammatory activation.3,4,9,40,50,70,71
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sonia Levi (University Vita-Salute San Raffaele, Milan, Italy) for the gift of anti-ferritin antibodies, CSL Behring for hemopexin and albumin purified from human sera, Tim Townes (University of Alabama at Birmingham, Birmingham, AL) for HbA and HbS sickle mice, Marieke Essers (German Cancer Research Center, Heidelberg, Germany) for TLR4-null mice for BMDM preparation, Richard Sparla and Andreas Simmelbauer for helping with some experiments, and Lucia De Franceschi (University of Verona) for critical reading of the manuscript. Microscope images were acquired at the Nikon Imaging Center at the University of Heidelberg, Heidelberg, Germany.
This work was supported by research funding from the Dietmar Hopp-Stiftung and the Deutsche Forschungsgemeinschaft (M.U.M.), Telethon grant GGP12082 (E.T.), a postdoctoral fellowship granted from the Medical Faculty of the University of Heidelberg (http://www.medizinische-fakultaet-hd.uni-heidelberg.de) (F.V.), and a grant by Fundação para a Ciência e Tecnologia, Portugal (M.C.d.S.).
Authorship
Contribution: F.V. and M.C.d.S. designed the project, performed the experiments, and wrote the manuscript; G.I. and S.P. contributed to some experiments; M.U.M, E.T., and A.C. designed and supervised the project and wrote the manuscript; and N.B. and A.Z. provided Hx and revised the manuscript.
Conflict-of-interest disclosure: N.B. and A.Z. are employees of CSL Behring. All other authors declare no competing financial interests.
Correspondence: Martina U. Muckenthaler, Department of Pediatric Oncology, Hematology and Immunology, Otto Meyerhof Zentrum, Im Neuenheimer Feld 350, 69120 Heidelberg, Germany; e-mail: martina.muckenthaler@med.uni-heidelberg.de; or Emanuela Tolosano, Molecular Biotechnology Center, Department of Molecular Biotechnology and Health Sciences, Via Nizza 52, 10126 Torino, Italy; e-mail: emanuela.tolosano@unito.it.
References
Author notes
F.V. and M.C.d.S. contributed equally to this work.
E.T. and M.U.M. contributed equally to this work.

![Figure 3. Heme induces macrophage polarization toward an M1-like phenotype, independently of the cell differentiation state. (A) M0, (B) M1, and (C) M2 BMDMs were left untreated (NT) or exposed to 5 μM heme-BSA (+Heme; 1:1 ratio) for 12 hours. M1 and M2 markers are shown in the left and right column of each panel, respectively. The expression of major histocompatibility complex class II (MHCII), CD86, CD14, and CD206 was analyzed by flow cytometry and is expressed in relative fluorescence units (RFU) as fold change compared with untreated cells (NT). The mRNA levels of TNFα, Arginase-1, Ym1, and IL-10 were analyzed by qRT-PCR and expressed in RQ, as fold change compared with untreated cells (NT). Results shown are the average of ≥3 independent experiments. (A direct comparison of expression levels of M1 and M2 markers in M0, M1, and M2 BMDMs is shown in supplemental Figure 7, where fold changes are compared with untreated [NT] M0 cells). (D) Analysis of the M1 markers MHCII and CD86 and the M2 marker CD206 and Arginase-1 in macrophages isolated from liver or spleen of untreated (NT) or heme-treated (hemin 30 μmol/kg) wild-type mice. Macrophage were analyzed 15 hours or 15 days after intravenous heme injection (n = 6). (E) Hematoxylin/eosin and Picrosirius red staining for collagen on liver sections from heme-treated wild-type mice (70 μmol/kg, 5 injections; scale bar, 300-100 μm). Arrows indicate infiltrates (hem/eo) and collagen (picrosirius). (F) Analysis of the M1 markers MHCII, CD86, TNFα, and IL-6 and the M2 marker CD206 in hepatic macrophages of control HbA and sickle HbS mice (n = 4). The expression of polarization markers in hepatic and splenic macrophages was analyzed by flow cytometry and is expressed in RFU as fold change compared with untreated wild-type mice (NT) or HbA controls. (G) Perls’ staining for iron on liver sections from HbA and HbS mice (scale bar, 100 μm). Values represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001. Regulation of iron-related genes in M0, M1, and M2 macrophages was monitored to control for the efficacy of heme and FeNTA treatment (supplemental Figure 9).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/4/10.1182_blood-2015-08-663245/4/m_473f3.jpeg?Expires=1769081134&Signature=sS8Oel3zTMiBBuxKNtf8D53Auq3cvy0HBrcGlu73I6eV6BcNOGU8A8Hhzm7LSuzWewrl5IqcSBdhKDFHXa2z-HiP86brJV4ZfMAcPPY-99dKOYVhSWsGnsFNp74jrgjwnLbPatqztnCOmMYRyXIeEGsb8IczVLbaY7Px2~qDluCSOmMJvW8PR0D5b4muBA3KnlpCaHyiNs67cYcfsQGEe-eUYg~6USqufrnyfI-dEGxtX0M37Ma0N3lzk9NSgR5nSE~BC2RQDPJQC3vpvhq4ggKgLNP7JYH5aA3bzfcv1tvSBRVOgTgQdufYtgJd72-mytQlQ6nzVOX7KYZoyjHU1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)