Key Points
A double-unit strategy does not decrease transplantation failure risk when a single unit of cord blood with adequate cell dose is available.
Alloreactivity may be enhanced by double-unit cord blood transplantation.
Abstract
Transplantation of 2 unrelated cord blood (UCB) units instead of 1 has been proposed to increase the cell dose. We report a prospective randomized study, designed to compare single- vs double-UCB transplantation in children and young adults with acute leukemia in remission or myelodysplasia. Eligible patients had at least two 4-6 HLA-identical UCBs with >3 × 107 nucleated cells/kg for the first and >1.5 × 107 for the second. The primary end point was the 2-year cumulative incidence of transplantation strategy failure, a composite end point including transplant-related mortality (TRM), engraftment failure, and autologous recovery. Randomized patients who did not proceed to transplantation due to refractory disease were considered transplantation failures. A total of 151 patients were randomized and included in the intent-to-treat analysis; 137 were transplanted. Double-UCB transplantation did not decrease transplantation strategy failure (23.4% ± 4.9% vs 14.9% ± 4.2%). Two-year posttransplant survival, disease-free survival, and TRM were 68.8% ± 6.0%, 67.6% ± 6.0%, and 5.9% ± 2.9% after single-unit transplantation compared with 74.8% ± 5.5%, 68.1% ± 6.0%, and 11.6% ± 3.9% after double-unit transplantation. The final relapse risk did not significantly differ, but relapses were delayed after double-unit transplantation. Overall incidences of graft-versus-host disease (GVHD) were similar, but chronic GVHD was more frequently extensive after double-UCB transplantation (31.9% ± 5.7% vs 14.7% ± 4.3%, P = .02). In an exploratory subgroup analysis, we found a significantly lower relapse risk after double-unit transplantation in patients receiving total body irradiation without antithymocyte globulin (ATG), whereas the relapse risk was similar in the group treated with busulfan, cyclophosphamide, and ATG. Single-UCB transplantation with adequate cell dose remains the standard of care and leads to low TRM. Double-unit transplantation should be reserved for patients who lack such units. This trial was registered at www.clinicaltrials.gov as #NCT01067300.
Introduction
During the last 2 decades, cryopreserved unrelated cord blood (UCB) has become an alternative stem cell source for patients who need hematopoietic stem cell transplantation (HSCT) and lack an HLA-identical donor.1-6 However, UCB units contain a limited number of hematopoietic cells and, as early as in the first reported clinical studies, this cell dose was identified as a major predictive factor for posttransplant outcome.1-4 Transplantation of 2 UCB units has been proposed to augment the transplanted cell dose with very promising early results, particularly in adult patients who frequently do not have a UCB unit with sufficient cell dose.7 In addition, several retrospective studies have suggested that transplantation of 2 UCB units induces the so-called graft-versus-graft (GVG) effect, leading to long-term engraftment of only 1 unit. The GVG effect may facilitate engraftment and promote more potent alloreactivity, ie, more graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) effect.8-12 In this context, randomized clinical trials were needed, but, for obvious ethical reasons as well as from a scientific point of view, such trials had to be performed only in patients with at least 1 identified UCB unit that contains a sufficient cell dose. If a GVG effect proved beneficial to these patients, double-unit UCB transplantation might no longer be reserved to those lacking an adequate cell dose containing UCB.
Wagner et al first published a randomized US study comparing 1- vs 2-unit UCB transplantation among children and adolescents with hematologic cancers. They found that 1-year survival rates were similar but that a double-unit cord-blood transplantation was associated with a higher risk of both acute and chronic GVHD.13 Despite a higher incidence of GVHD, an enhanced GVL effect was not observed after double-unit transplantation, a finding that was in contrast to that in some previous retrospective studies.9,12
We present here the results of a multicenter French randomized study, comparing single vs double-unit unrelated cord blood transplantation (UCBT) after myeloablative conditioning regimen in children, adolescents, and young adults younger than 35 years with either acute leukemia in remission or myelodysplastic syndrome (MDS).
Methods
Inclusion criteria
Patients were eligible for this study if they met all the following criteria: (1) age <35 years, (2) acute leukemia in complete remission or MDS with <20% bone marrow blasts, (3) requiring unrelated HSCT, and (4) absence of an unrelated donor considered to be acceptable by the transplant center on the basis of HLA compatibility and donor availability. In addition, eligible patients had at least 2 UCBs that were 4-6 HLA identical to the patient and between them contained >3 × 107 total nucleated cells (TNCs) per kilogram of recipient body weight for the first unit and >1.5 × 107 TNCs/kg for the second.
Patients with a history of allogeneic stem cell transplantation or with a poor health status who, according to the transplant center evaluation, precluded the use of a myeloablative conditioning regimen were excluded. All patients (or their parents or legal guardians) provided written informed consent. This study was approved by the French National Program for Clinical Research and the French National Cancer Institute. This trial was registered at www.clinicaltrials.gov as #NCT01067300.
Transplantation procedures
Cord blood transplant characterization and selection.
Donor-recipient HLA matching was assessed at low- to intermediate-resolution level molecular typing for HLA-A and HLA-B loci and high-resolution genotyping for the HLA-DRB1 locus. HLA compatibility was expressed as the number of identical loci out of 6. All selected UCB had to fulfill the eligibility criteria detailed above. When several units were available, a 6/6 HLA-identical unit was preferred to a 5/6 HLA-identical and a 5/6 unit to a 4/6. At the same number of HLA mismatches, an A or B difference was preferred to a DRB1 mismatch. At the same overall HLA compatibility level, the unit with the higher TNC count was selected.
Conditioning regimen and GVHD prophylaxis
All patients received a myeloablative conditioning regimen with busulfan, cyclophosphamide, and antithymocyte globulin (BuCyATG) if they were younger than 4 years or fludarabine, total body irradiation (TBI), and cyclophosphamide (FluTBICy) if they were older. BuCyATG was mandatory for children younger than 4 years and could also be used for older patients with AML, according to the policy of each transplant center. The BuCyATG regimen included busulfan from day −9 to day −6 with a dosage depending on the recipient’s weight (<9 kg: 1 mg/kg × 4/d; from 9 to 16 kg: 1.2 mg/kg × 4/d; from 16 to 23 kg: 1.1 mg/kg × 4/d), 50 mg/kg cyclophosphamide per day from day −5 to day −2, and 2.5 mg/kg rabbit antithymocyte globulin (ATG) per day from day −3 to day −1. The FluTBICy included 25 mg/m2 fludarabine per day from day −9 to day −7, TBI from day −6 to day −4, and 60 mg/kg cyclophosphamide per day from day −3 to day −2. TBI was fractionated over 3 days with 2 Gy twice a day for a 12 Gy cumulative dose with lung shielding at 8 Gy. Busulfan pharmacokinetic monitoring was not mandatory. Granulocyte colony-stimulating factor was systematically given, beginning on day +5, and continued until the blood neutrophil count reached 1 g/L.
GVHD prophylaxis
GVHD prophylaxis consisted of cyclosporine A and steroid after BuCyATG and cyclosporine A and mycophenolate mofetil after FluTBICy. Cyclosporine A was given at full dosage from day −3 to day +100, then tapered and stopped at day +180 in patients with undetectable pretransplant minimal residual disease (MRD) without posttransplant GvHD and who achieved complete donor chimerism. Its duration was modulated according to the pre- and posttransplant level of MRD as well as to GVHD and chimerism. Mycophenolate mofetil (15 mg/kg × 3/d) was administered from day −3 to day +30.
Study end points and statistical methods
End point definitions.
The primary end point was the cumulative incidence of “transplantation strategy failure.” Transplantation failure corresponded either to transplant-related mortality (TRM) or to primary engraftment failure that did not result in death because a second transplantation was performed or because autologous recovery occurred. Thus, “transplantation failure” was defined as the first of the 4 following events: TRM, autologous recovery (defined as hematopoietic recovery with >80% blood recipient chimerism), second allogeneic transplantation, or infusion of an autologous stem cell rescue for engraftment failure. In an intention-to-treat analysis, patients who relapsed between randomization and their planned transplantation and could never be transplanted in remission due to refractory disease were also considered as transplantation strategy failure. Cumulative incidence was calculated from the day of randomization.
Secondary end points included the main posttransplant outcomes: hematologic recovery, relapse risk, incidence and grading of acute and chronic GVHD, immune recovery, TRM, disease-free survival (DFS), and overall survival. Neutrophil engraftment date was defined as the first of 3 consecutive days with >0.5 g/L blood neutrophils. Platelet engraftment was the first of 3 consecutive days with >20 g/L blood platelets in the absence of platelet transfusion during the last 7 days. Lymphocyte subpopulation recovery was analyzed in peripheral blood by flow cytometry at 1, 3, 6, 12, and 24 months. Several immunologic end points were chosen for their potential clinical significance: time to achieve a CD4+ cell count >200/mm3, CD4+ >500/mm3, CD8+ >250/mm3, CD3+ >500/mm3, CD3+ >1000/mm3, and CD19 > 200/mm3. Diagnosis and grading for GVHD were performed according to published criteria.14,15
Sample size calculation and statistical methods
The primary objective of the study was to compare the cumulative incidence of transplantation failure between the 2 study arms. Sample size calculation was carried out on the basis of this primary end point according to the multiple testing procedure method published by O’Brien and Fleming. In the setting of a sequential trial including 1 interim and 1 final analysis, with the cumulative incidence of transplantation failure hypothesis being 40% in the single-unit arm and 20% in the double-unit arm (α risk, 5%, power, 80%), the estimate of sample size was 76 by group, ie, a total of 152 patients.
The randomization was stratified using the method of randomly permuted blocks.16 The strata were age groups (0-9 years, 10-24 years, and 25-35 years), TBI (yes/no), and type of blood disease (lymphoid/myeloid). The interim analysis was performed as planned and submitted to 3 independent experts who concluded that the study could continue until completion. The final analysis includes data collected as of September 2015. The cumulative incidence function with competing events was used to estimate transplantation failure.17 Comparisons were done using the Fine and Gray model.18 The competing risk for transplantation failure was relapse. The same method was used to evaluate relapse risk, TRM, neutrophil and platelet engraftment, T and B lymphocyte subset recovery, and acute and chronic GVHD. The probabilities of overall survival and DFS were calculated using the Kaplan-Meier method and compared with the log-rank test.19 All probabilities are given at 2 years and provided with their 95% confidence interval.
Results
Patient, disease, and transplant characteristics
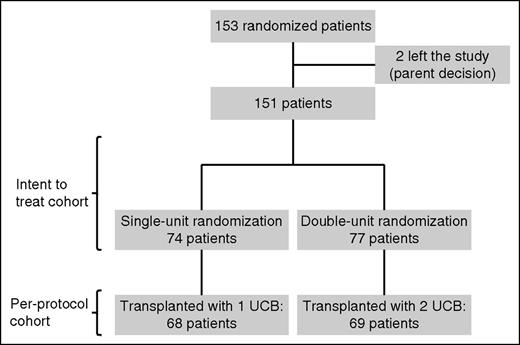
Between February 2010 and February 2015, 153 patients were randomized in 22 French transplantation centers. As shown in Figure 1, 2 patients left the study after randomization by parent decision and were excluded from the analysis. All of the other 151 patients were included in the intent-to-treat analysis: 74 were randomly assigned to the single-unit arm and 77 to the double-unit arm. A total of 14 out of 151 patients (6 in the single-unit arm and 8 in the double-unit arm) relapsed between randomization and their planned graft and could never be transplanted in remission because of refractory disease. None of the 137 transplanted patients randomly assigned to receive a single- or double-unit transplant crossed over to the other treatment group.
Cumulative incidence of transplantation failure. Intent-to treat analysis.
As shown in Table 1, the 2 treatment arms were well balanced with respect to sex, age, diagnosis, hematologic status at randomization, conditioning regimen, time interval from randomization to transplant, and follow-up duration. Overall, 79.5% of the patients were <18 years, 59.6% had acute lymphocytic leukemia (ALL), 40.4% had acute myeloid leukemia (AML) or MDS, 48.3% were in first complete remission at the time of randomization, 60.6% received FluTBICy, and 39.4% received BuCyATG. The mean interval from randomization to transplantation was 39.3 ± 1.4 days. The mean follow-up period from transplantation was 798.1 ± 46.9 days. As expected, mean age at transplantation was higher in the FluTBICy group than in the BuCyATG (13.8 vs 6.7 years, P < 10−3). Similarly, 21.7% of patients who received FluTBICy had AML/MDS and 78.3% had ALL, whereas in the BuCyATG group, 72.2% had AML/MDS and 27.8% had ALL.
Patient characteristics according to randomization
| . | Single-unit UCB (n = 74) . | Double-unit UCB (n = 77) . | P . |
|---|---|---|---|
| Sex | .84 | ||
| Male | 45 (60.8) | 48 (62.3) | |
| Female | 29 (39.2) | 29 (37.7) | |
| Age | .90 | ||
| 0-9 y | 39 (52.7) | 43 (55.8) | |
| 10-24 y | 28 (37.8) | 28 (36.4) | |
| 25-35 y | 7 (9.5) | 6 (7.8) | |
| Age | .94 | ||
| <18 y | 59 (79.7) | 61 (79.2) | |
| ≥18 y | 15 (20.3) | 16 (20.8) | |
| Diagnosis | .77 | ||
| ALL | 45 (60.8) | 45 (58.4) | |
| AML/MDS | 29 (39.2) | 32 (41.6%) | |
| Hematologic status at randomization | .10 | ||
| CR 1 | 31 (41.9) | 42 (54.5) | |
| CR ≥2 | 41 (55.4) | 30 (39.0) | |
| MDS | 2 (2.7) | 5 (6.5) | |
| Transplanted patients | |||
| Conditioning regimen | n = 68 | n = 69 | |
| FluTBICy | 41 (60.3) | 42 (60.9) | .94 |
| BuCyATG | 27 (39.7) | 27 (39.1) | |
| Interval from randomization to HSCT (days) | |||
| Mean ± SEM | 38.9 ± 1.9 | 39.7 ± 2.1 | .20 |
| Median (range) | 35.5 (13-87) | 34 (17-83) | .78 |
| Follow-up duration from HSCT (days) | |||
| Mean ± SEM | 781 ± 66.5 | 815 ± 66.6 | .71 |
| Median (range) | 709 (15-1930) | 800 (24-1814) | .73 |
| . | Single-unit UCB (n = 74) . | Double-unit UCB (n = 77) . | P . |
|---|---|---|---|
| Sex | .84 | ||
| Male | 45 (60.8) | 48 (62.3) | |
| Female | 29 (39.2) | 29 (37.7) | |
| Age | .90 | ||
| 0-9 y | 39 (52.7) | 43 (55.8) | |
| 10-24 y | 28 (37.8) | 28 (36.4) | |
| 25-35 y | 7 (9.5) | 6 (7.8) | |
| Age | .94 | ||
| <18 y | 59 (79.7) | 61 (79.2) | |
| ≥18 y | 15 (20.3) | 16 (20.8) | |
| Diagnosis | .77 | ||
| ALL | 45 (60.8) | 45 (58.4) | |
| AML/MDS | 29 (39.2) | 32 (41.6%) | |
| Hematologic status at randomization | .10 | ||
| CR 1 | 31 (41.9) | 42 (54.5) | |
| CR ≥2 | 41 (55.4) | 30 (39.0) | |
| MDS | 2 (2.7) | 5 (6.5) | |
| Transplanted patients | |||
| Conditioning regimen | n = 68 | n = 69 | |
| FluTBICy | 41 (60.3) | 42 (60.9) | .94 |
| BuCyATG | 27 (39.7) | 27 (39.1) | |
| Interval from randomization to HSCT (days) | |||
| Mean ± SEM | 38.9 ± 1.9 | 39.7 ± 2.1 | .20 |
| Median (range) | 35.5 (13-87) | 34 (17-83) | .78 |
| Follow-up duration from HSCT (days) | |||
| Mean ± SEM | 781 ± 66.5 | 815 ± 66.6 | .71 |
| Median (range) | 709 (15-1930) | 800 (24-1814) | .73 |
Data are presented as n (%) of patients, unless otherwise indicated.
CR, complete remission.
Graft characteristics according to treatment group among patients who underwent transplantation are detailed in Table 2. As planned, all transplanted UCB units were 4-6/6 HLA identical to the recipient and 4-6/6 HLA identical to the other UCB in case of double-unit transplantation. The mean values of cryopreserved and infused TNCs per kilogram of recipient weight were 6.3 ± 0.5 and 4.7 ± 0.4 in the single-unit arm and 12.2 ± 0.9 and 9.1 ± 0.8 in the double-unit arm, respectively.
UCB characteristics in transplanted patients only
| . | Double-unit UCB (n = 69) . | Single-unit UCB (n = 68) . | ||
|---|---|---|---|---|
| UCB-1 . | UCB-2 . | Combined . | ||
| HLA compatibility, n (%) | ||||
| 4/6 | 16 (23.2) | 18 (26.1) | 23 (33.3)* | 19 (27.9) |
| 5/6 | 48 (69.6) | 48 (69.6) | 45 (65.2)* | 36 (53.0) |
| 6/6 | 5 (7.2) | 3 (4.3) | 1 (1.5)* | 13 (19.1) |
| Cell doses, mean ± SEM | ||||
| Cryopreserved | ||||
| TNCs (× 107/kg) | 6.2 ± 0.5 | 5.9 ± 0.5 | 12.2 ± 0.9 | 6.3 ± 0.5 |
| CD34+ cells (× 105/kg) | 2.7 ± 0.2 | 2.2 ± 0.2 | 4.9 ± 0.4 | 2.6 ± 0.3 |
| Infused | ||||
| TNCs (× 107/kg) | 4.6 ± 0.4 | 4.6 ± 0.5 | 9.1 ± 0.8 | 4.7 ± 0.4 |
| CD34+ cell (× 105/kg) | 1.9 ± 0.2 | 1.5 ± 0.2 | 3.3 ± 0.3 | 1.9 ± 0.2 |
| CD45+ cell (× 107/kg) | 4.5 ± 1.1 | 3.9 ± 0.6 | 8.1 ± 1.4 | 4.1 ± 0.6 |
| CD3+ cell (× 106/kg) | 8.7 ± 0.9 | 8.5 ± 1.1 | 17.1 ± 1.6 | 7.8 ± 0.9 |
| . | Double-unit UCB (n = 69) . | Single-unit UCB (n = 68) . | ||
|---|---|---|---|---|
| UCB-1 . | UCB-2 . | Combined . | ||
| HLA compatibility, n (%) | ||||
| 4/6 | 16 (23.2) | 18 (26.1) | 23 (33.3)* | 19 (27.9) |
| 5/6 | 48 (69.6) | 48 (69.6) | 45 (65.2)* | 36 (53.0) |
| 6/6 | 5 (7.2) | 3 (4.3) | 1 (1.5)* | 13 (19.1) |
| Cell doses, mean ± SEM | ||||
| Cryopreserved | ||||
| TNCs (× 107/kg) | 6.2 ± 0.5 | 5.9 ± 0.5 | 12.2 ± 0.9 | 6.3 ± 0.5 |
| CD34+ cells (× 105/kg) | 2.7 ± 0.2 | 2.2 ± 0.2 | 4.9 ± 0.4 | 2.6 ± 0.3 |
| Infused | ||||
| TNCs (× 107/kg) | 4.6 ± 0.4 | 4.6 ± 0.5 | 9.1 ± 0.8 | 4.7 ± 0.4 |
| CD34+ cell (× 105/kg) | 1.9 ± 0.2 | 1.5 ± 0.2 | 3.3 ± 0.3 | 1.9 ± 0.2 |
| CD45+ cell (× 107/kg) | 4.5 ± 1.1 | 3.9 ± 0.6 | 8.1 ± 1.4 | 4.1 ± 0.6 |
| CD3+ cell (× 106/kg) | 8.7 ± 0.9 | 8.5 ± 1.1 | 17.1 ± 1.6 | 7.8 ± 0.9 |
The combined match score for patients in the double-unit UCB group is the minimum HLA match between the first unit and the recipient or the second unit and the recipient. For example, if UCB-1 was a 6/6 match with the recipient and UCB-2 was a 4/6 match, the match score assigned for that recipient was 4/6.
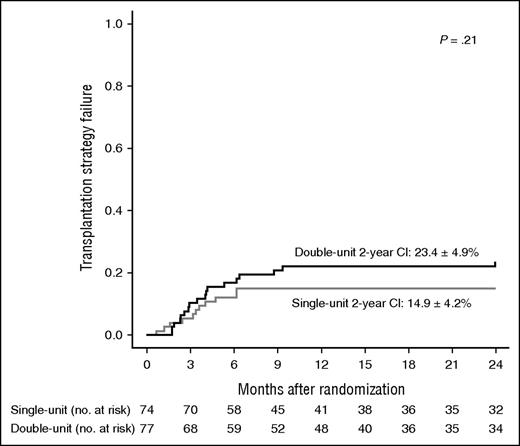
Primary end point analysis
The randomization arm did not statistically influence the 2-year cumulative incidence of transplantation strategy failure (Figure 2). In the intent-to-treat analysis (ie, when patients who relapsed before their planned graft and could never be transplanted due to refractory disease were considered transplantation failures), the cumulative incidence was 14.9% ± 4.2% in the single-unit arm and 23.4% ± 4.9% in the double-unit arm (P = .21). In the per-protocol analysis (ie, when only transplanted patients were considered), the incidence was 7.3% ± 3.2% vs 14.5% ± 4.3%, respectively (P = .20). For 5 patients who experienced transplantation strategy failure after receiving single-unit UCB, the first classifying event was TRM in 3, autologous recovery in 1, and second transplant for engraftment failure in 1. For 10 patients who had transplantation strategy failure after receiving double-unit UCB, the first classifying event was TRM in 8, autologous recovery in 1, and second transplant for engraftment failure in 1.
Secondary end point analysis
Hematologic recovery was similar after transplantation of either 1 or 2 UCB units. The median time to neutrophil recovery was 24.8 ± 1.0 days after single-unit and 23.5 ± 0.8 days after double-unit transplantation, the day +60 probability of recovery being 92.6% ± 3.3% and 94.2% ± 3.0%, respectively. For platelet recovery, the median time was 58.1 ± 3.8 days vs 55.9 ± 3.3 days, respectively.
The main posttransplant outcomes according to treatment group are provided in Table 3. The overall 2-year cumulative incidence of relapse did not significantly differ between the 2 treatment groups (Figure 3A), but relapses occurred earlier in the single-unit arm. The mean interval from transplantation to relapse was 282.4 ± 48 days after double-unit transplantation compared with 164.9 ± 17.6 days after single-unit transplantation (P = .04). The overall incidences of acute and chronic GvHD did not differ, but chronic GVHD was more frequently extensive after double-unit UCBT. The 2-year cumulative incidence of extensive chronic GVHD was 31.9% ± 5.7% after double-unit vs 14.7% ± 4.3% after single-unit transplantation (P = .02). Probabilities of 2-year overall survival, DFS, and TRM were not different (68.8% ± 6.0%, 67.6% ± 6.0%, and 5.9% ± 2.9% after single-unit transplantation vs 74.8% ± 5.5%, 68.1% ± 6.0%, and 11.6% ± 3.9% after double-unit transplantation). The 2-year probability of DFS without having experienced any extensive chronic GVHD was 56.0% ± 6.3% after single UCBT and 41.8% ± 6.4% after double UCBT (P = .15). A total of 35 patients died after transplantation (19 in the single-unit arm and 16 in the double-unit arm). Causes of death after single-unit transplantation were disease-related relapse in 15 patients, engraftment failure in 2, infectious complication in 1, and veno-occlusive disease in 1. After double transplantation, causes of death included relapse in 8 patients, infectious complication in 5, adult respiratory distress in 1, GVHD in 1, and thrombotic microangiopathy in 1. As shown in supplemental Table 1 (available on the Blood Web site), the recovery rates for T- and B-cell subpopulations were similar between treatment arms.
Secondary end points: cumulative incidence of main posttransplant outcomes
| . | Single-unit UCB (n = 68) . | Double-unit UCB (n = 69) . | P . |
|---|---|---|---|
| 2-y CI of relapse | 23.5% ± 5.2% | 17.4% ± 4.6% | .31 |
| Day 100 acute GVHD | |||
| Grade ≥2 | 41.2% ± 6.0% | 44.9% ± 6.0% | .76 |
| Grade ≥3 | 25.0% ± 5.3% | 18.8% ± 4.7% | .40 |
| 2-y chronic GVHD | |||
| Overall | 50.0% ± 6.8% | 52.6% ± 6.7% | .70 |
| Extensive only | 14.7% ± 4.3% | 31.9% ± 5.7% | .02 |
| 2-y TRM | 5.9% ± 2.9% | 11.6% ± 3.9% | .25 |
| 2-y DFS | 67.6% ± 6.0% | 68.1% ± 6.0% | .74 |
| 2-y overall survival | 68.8% ± 6.0% | 74.8% ± 5.5% | .56 |
| . | Single-unit UCB (n = 68) . | Double-unit UCB (n = 69) . | P . |
|---|---|---|---|
| 2-y CI of relapse | 23.5% ± 5.2% | 17.4% ± 4.6% | .31 |
| Day 100 acute GVHD | |||
| Grade ≥2 | 41.2% ± 6.0% | 44.9% ± 6.0% | .76 |
| Grade ≥3 | 25.0% ± 5.3% | 18.8% ± 4.7% | .40 |
| 2-y chronic GVHD | |||
| Overall | 50.0% ± 6.8% | 52.6% ± 6.7% | .70 |
| Extensive only | 14.7% ± 4.3% | 31.9% ± 5.7% | .02 |
| 2-y TRM | 5.9% ± 2.9% | 11.6% ± 3.9% | .25 |
| 2-y DFS | 67.6% ± 6.0% | 68.1% ± 6.0% | .74 |
| 2-y overall survival | 68.8% ± 6.0% | 74.8% ± 5.5% | .56 |
CI, cumulative incidence.
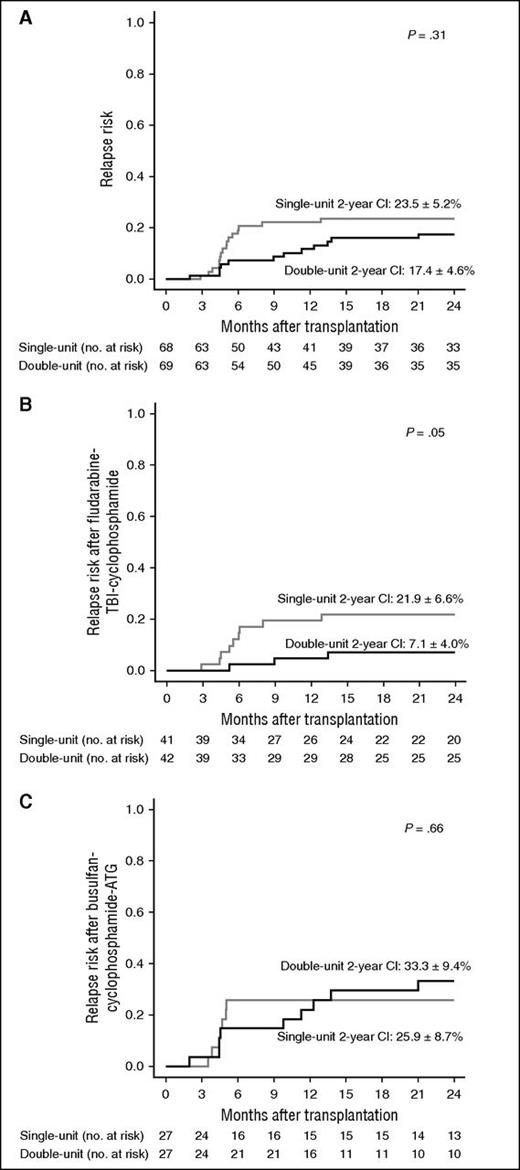
Cumulative incidence of posttransplant relapse. (A) Overall cohort. (B) After fludarabine-TBI-cyclophosphamide. (C) After busulfan-cyclophosphamide and ATG.
Cumulative incidence of posttransplant relapse. (A) Overall cohort. (B) After fludarabine-TBI-cyclophosphamide. (C) After busulfan-cyclophosphamide and ATG.
An exploratory subgroup analysis according to the 2 transplantation procedures was performed separately. In the subgroup of patients who received FluTBICy as a conditioning regimen and cyclosporine A and mycophenolate mofetil as GVHD prophylaxis, the relapse risk was reduced after double UCBT when compared with single UCBT (7.1% ± 4.0% vs 21.9% ± 6.6%, P = .05; Figure 3B). By contrast, relapse risk was similar in the 2 treatment groups for patients who received busulfan-cyclophosphamide and ATG as conditioning regimen and cyclosporine A and steroid as GVHD prophylaxis (Figure 3C). In the TBI subgroup, the lower relapse risk associated with double-unit transplantation did not translate into a significantly better survival (79.8% ± 6.4% vs 71.4% ± 7.8% in the single arm) due to a trend toward higher TRM (14.3% ± 5.5% vs 2.4% ± 2.4%, P = .06). Similarly, the effect of randomization was analyzed separately in AML/MDS and ALL. We did not observe any significant differences, although there was a trend toward a lower relapse risk after double UCB in the ALL group (12.2% ± 5% vs 25.6% ± 7%, P = .1).
Finally, we also analyzed the main posttransplant outcomes according to the degree of UCB/recipient HLA compatibility (see supplemental Table 2). In the single-unit arm, the only significant difference was the 2-year TRM, which was 2% ± 2% after a 5-6/6 HLA-identical UCBT compared with 15.8% ± 8.6% after a 4/6 HLA-compatible UCBT (P = .03). However, this did not translate into better overall results, with the 2-year survival being 69% ± 7.2% and 68% ± 10.8% in the 5-6/6 and 4/6 groups, respectively. In the double-unit arm, there was no significant difference according to the first unit/recipient HLA compatibility. Survival at 2 years was 74.4% ± 6.7% in the 5-6/6 group and 75.8% ± 9.8% for a 4/6 HLA match.
Discussion
This prospective, multicenter, and randomized study was designed to compare the results of single vs double UCBT in children and young adults with acute leukemia or MDS. Wagner et al, who recently reported a randomized study on the same subject, showed that double-unit UCBT was unable to improve the 1-year survival probability when a single unit had sufficient cell dose and HLA compatibility.13 Although our primary end point (ie, the 2-year cumulative incidence of transplantation strategy failure) was somewhat different, we also conclude that, overall, results of double-unit transplantation are similar to those of single-unit transplantation. Indeed, overall survival 2 years after transplantation was 74.8% in our double-unit arm and 68.8% in the single-unit arm, a difference that was not significant. Noteworthy, our primary end point was designed to take into consideration, in addition to TRM, other adverse transplantation events such as engraftment failure that did not result in death due to autologous recovery or second allogeneic transplantation. In our intent-to-treat analysis, we also considered transplantation strategy failure patients who relapsed after randomization, but before their planned graft, and who could never be transplanted in remission due to refractory disease. Using this end point, we also demonstrate that double-unit transplantation is ineffective at improving outcome when a single unit of UCB containing >3 × 107 TNCs/kg can be used.
The incidence of any degree of chronic GVHD at 2 years was similar in our 2 treatment arms, but we found a higher incidence of extensive disease after double-unit transplantation. This is concordant with Wagner et al, who demonstrated exactly the same feature. In their study, however, despite the higher incidence of GVHD, an enhanced GVL effect was not observed after double-unit transplantation. The 1-year relapse they reported was similar and remarkably low at 14% and 12% after single- and double-unit UCBT, respectively. This finding is in contrast to that reported on several previous retrospective studies.9,12,20
Although alloreactivity and relapse risk were not the primary end points of our trial, some exploratory analyses suggest that the GVL effect may be enhanced by transplantation of 2 units instead of 1 unit. Firstly, relapses occurred later in the double-unit arm: the mean interval from transplantation to relapse was 282.4 days after double-unit transplantation vs 164.9 after single-unit transplantation (P = .04). Moreover, 2 transplantation procedures were used in our study: one included TBI and no ATG (FluTBICy), and the other included busulfan cyclophosphamide with ATG (BuCyATG). There was a significantly lower relapse risk after double-unit transplantation in the FluTBICy group (7.1% vs 21.9%), whereas the relapse risk was similar in the group with ATG. One could argue that ATG, and probably other very effective GVHD prophylaxis, may suppress a potentially higher GVL effect of double-unit transplantation. On the other hand, relapse risk after HSCT in a given cohort is the final result of complex interactions, which also include the disease itself and MRD at the time of transplantation. We cannot exclude that heterogeneity in MRD status at the time of UCBT could also explain the difference observed between our study and the previously published randomized study.
Nevertheless, even with a lower relapse risk in the FluTBICy group, double-unit UCBT did not confer a statistically significant survival advantage, the 2-year overall survival being 79.8% after double-unit UCBT in this subgroup vs 71.4% after single-unit UCBT. Consequently, single-unit UCBT has to remain the standard of care if a 4-6 HLA-identical unit that contains >3 × 107 TNCs/kg is identified, and double-unit UCBT should be reserved for patients who lack a UCB unit with sufficient cell dose. Beyond the results of the randomization, this prospective multicenter study also pointed out the results that can be achieved after myeloablative conditioning and single-unit UCBT in children and young adults with hematologic malignancies. The 2-year TRM was 5.9% in this study after single-unit UCBT, which appears less than reported in many older studies and is clearly less than we expected at the time of study initiation. Noteworthy, among the 4 patients who died of transplant-related complications after single UCBT, only 2 were due to engraftment failure. Explanations for this apparent improvement are probably multifactorial and may include enhanced quality of selected cord blood units (notably for the cell dose), as well as improvement in the treatment regimen and supportive care. Finally, overall 2-year survival was 68.8% after single-unit UCBT and 74.8% after double-unit UCBT. One could argue that the results in the single-unit arm may have been favorably influenced by a relatively high proportion of 6/6 HLA-identical UCB in the single-unit arm (19.1% vs 7.2%; Table 2). However, as shown in “Results,” the degree of UCB/recipient HLA compatibility did not apparently influence overall survival in this study.
We conclude that a double-unit strategy proved ineffective at improving the outcome of a UCB transplant when a single cord has an adequate cell dose. Future approaches to facilitate engraftment and improve patient survival may include better HLA matching with high-resolution typing of 8 instead of 6 loci,21 as well as in vivo cord blood priming or expansion.22-25
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients and their families for participating in the study.
The authors are very grateful to Guy Leverger (Department of Pediatric Hematology/Oncology, Trousseau Hospital, Paris) and Norbert Vey (Department of Hematology-Oncology, Paoli-Calmettes Institute [IPC], Marseille), who had a very active role in the interim analysis as independent experts; and Lilian Laborde (management and analysis center IPC-PACA, Marseille) and Junia V. Melo (University of Adelaide, Australia, and Imperial College, London) for medical editing of the manuscript.
The authors thank the following investigators from different centers who included patients: P. S. Rohrlich and F. Larosa (Pediatric Hematology, Saint Jacques Hospital, Besançon); F. Legrand, F. Larosa, and E. Deconinck (Hematology, Jean Minjoz Hospital, Besançon); R. Tabrizi and S. Vigouroux (Hematology, Haut-Leveque Hospital, Bordeaux); S. Ducassou (Pediatric Hematology-Oncology, Pellegrin Hospital, Bordeaux); E. Doré, J. Kanold, F. Demeocq, E. Merlin, and F. Isfan (Pediatric Hematology-Oncology, Hôtel Dieu Hospital, Clermont-Ferrand); J. O. Bay, V. Cacheux, R. Guièze, C. Chabrot, E. Hermet, O. Tournilhac, B. De Renzis, and C. Chateleix (Hematology, Estaing Hospital, Clermont-Ferrand); C. E. Bulabois, A. Thiebaut, J. Y. Cahn, and F. Garban (Hematology-Oncology, Albert Michallon Hospital, Grenoble); D. Plantaz, S. Bobillier, D. Adjaoud, A. Pagnier, L. Cotta, C. Armari-Alla, and P. Girard (Pediatric Hematology-Oncology, Albert Michallon Hospital, Grenoble); V. Coiteux and R. Dulery (Department of Hematology, Huriez Hospital, Lille); H. Labussière, M. Detrait, F. Barraco, S. Ducastelle-Lepretre, F. Nicolini, and X. Thomas (Department of Hematology, Edouard Herriot Hospital, Lyon); V. Mialou (Institute of Pediatric Hematology-Oncology, Lyon); S. Furst, J. El-Cheikh, R. Crocchiolo, and L. Castagna (Department of Hematology-Oncology, IPC, Marseille); G. Margueritte (Department of Pediatric Hematology-Oncology, Montpellier); P. Bordigoni, A. Salmon, and L. Clément (Department of Pediatric Hematology-Oncology, Nancy); N. Dhédin, J. P. Vernant, S. Nguyen, and M. Uzunov (Department of Hematology, AP-HP, La Pitié Salpétrière Hospital, Paris); G. Socié, R. Peffault de Latour, P. Ribaud, A. Xhaard, M. Robin, N. Dhédin, and F. Sicre de Fontbrune (Department of Hematology, AP-HP, Saint Louis Hospital, Paris); K. Yakouben, F. Duquesne, J. Lachenaud, A. Baruchel, M. Ouachée, E. Serror, B. Lescoeur, and M. Fahd (Department of Pediatric Hematology, AP-HP, Robert Debré Hospital, Paris); F. Toutain, S. Taque, and C. Chappé (Department of Pediatric Hematology-Oncology, Rennes); P. Schneider, A. Marie-Cardine, C. Dumesnil, and N. Buchbinder (Department of Pediatric Hematology-Oncology, Rouen); and C. Paillard (Department of Pediatric Hematology-Oncology, Strasbourg).
This work was supported by the French National Institute of Cancer, the French Society of Hematopoietic Stem Cell Transplantation, and the French Society of Childhood Cancer.
Authorship
Contribution: G.M. designed research, performed research, analyzed data, and wrote the manuscript; C.G. and J.-H.D. designed research, performed research, and analyzed data; A.L. and K.B. designed research and performed statistical analysis; and A.S., C.P., B.B., C.J., I.Y.-A., N.M., P.L., A.M.-C., V.G., D.B., M. Michallet, F.R., C.R., C.O., S.E., M.S., M. Mohty, and V.R. contributed to research and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gérard Michel, Department of Pediatric Hematology Oncology, Hôpital d’Enfants La Timone and Université Aix-Marseille, Marseille, France; e-mail: gmichel@ap-hm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal