Key Points
Concomitant PI3Kδ and SYK inhibition resulted in treatment-emergent pneumonitis, necessitating early study termination.
Initial trials of novel combinations should use conservative designs that are focused on safety.
Abstract
Although agents targeting B-cell receptor signaling have provided practice-changing results in relapsed chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL), they require prolonged administration and provide incomplete responses. Given synergistic preclinical activity with phosphatidylinositol 3-kinase δ and spleen tyrosine kinase inhibition, this phase 2 study evaluated the safety and efficacy of the combination of idelalisib and entospletinib. Eligible patients with relapsed or refractory CLL or NHL underwent intrapatient dose escalation with each agent. With a median treatment exposure of 10 weeks, 60% and 36% of patients with CLL or follicular lymphoma, respectively, achieved objective responses. However, the study was terminated early because of treatment-emergent pneumonitis in 18% of patients (severe in 11 of 12 cases). Although most patients recovered with supportive measures and systemic steroids, 2 fatalities occurred and were attributed to treatment-emergent pneumonitis. Increases of interferon-γ and interleukins 6, 7, and 8 occurred over time in patients who developed pneumonitis. Future studies of novel combinations should employ conservative designs that incorporate pharmacodynamics/biomarker monitoring. These investigations should also prospectively evaluate plasma cytokine/chemokine levels in an attempt to validate biomarkers predictive of response and toxicity. This trial was registered at www.clinicaltrials.gov as #NCT01796470.
Introduction
Agents targeting pathways downstream of the B-cell receptor (BCR) involving phosphatidylinositol 3-kinase (PI3K), Bruton tyrosine kinase, and spleen tyrosine kinase (SYK) are redefining standard-of-care treatment options for patients with lymphoid malignancies. However, responses are incomplete, and residual disease remains difficult to eliminate. Simultaneous inhibition of multiple kinases in the BCR signaling pathway can produce synergistic antitumor activity in vitro, suggesting the potential to improve the depth of clinical responses and to overcome treatment resistance.
To improve on single-agent results, we investigated the combination of idelalisib, a selective inhibitor of PI3Kδ, and entospletinib, a small-molecule inhibitor specific to SYK.1,2 In vitro, the combination synergistically decreased the viability of primary chronic lymphocytic leukemia (CLL) cells, including those harboring the 17p deletion.3 Further, chemotactic chemokine signaling was disrupted to a significantly greater degree compared with that observed with either agent alone. The combination of idelalisib and entospletinib was administered to healthy volunteers for 1 month using 3 different dose levels of each drug. Treatment was well tolerated, and no clinically relevant changes in idelalisib or entospletinib pharmacokinetics were observed.4 We conducted a phase 2 clinical trial combining idelalisib and entospletinib in patients with CLL or non-Hodgkin lymphoma (NHL).
Study design
To evaluate the combination of idelalisib and entospletinib, 5 cohorts consisting of patients with indolent NHL, CLL, mantle-cell lymphoma, or diffuse large B-cell lymphoma (DLBCL) were studied concurrently in an open-label study. Each patient underwent dose escalation of idelalisib and entospletinib (from 100 to 150 mg and 200 to 800 mg twice daily, respectively) every 2 or 4 weeks (supplemental Table 1, available on the Blood Web site). The primary efficacy end point was objective response rate per standard NHL and CLL response criteria.5,6 Protocols were approved by institutional review boards at each participating site, and all patients signed written informed consent prior to participation.
Assessments
Response assessments were conducted every 8 weeks during the first 24 weeks on study and then every 12 weeks thereafter. The severity of adverse events (AEs) was graded according to the Common Terminology Criteria for Adverse Events, Version 4.03. To evaluate the biologic effects of the combination therapy, blood samples were obtained at baseline and weeks 1, 2, 3, 4, 6, 8, 12, 16, and 20. Analysis by Luminex immunoassays (Millipore, Billerica, MA) was performed using serum samples for cytokine/chemokine parameters (interferon-γ, interleukin 6 [IL-6], IL-7, and IL-8) to better understand the immune etiology of pneumonitis.
Statistical methods
The objective response rate and 90% exact confidence interval were conducted in the full analysis set based on independent review committee assessments. Changes in cytokine/chemokine from baseline were analyzed for patients in this combination study who experienced pneumonitis, those who did not, and patients treated in the phase 2 entospletinib monotherapy study.
Results and discussion
Sixty-six patients were enrolled prior to trial suspension. Baseline demographics are summarized in supplemental Table 2. Histologies enrolled included CLL (n = 35), follicular lymphoma (n = 14), DLBCL (n = 6), mantle-cell lymphoma (n = 3), small lymphocytic lymphoma (n = 3), marginal zone lymphoma (n = 3), and lymphoplasmacytic lymphoma (n = 2). The median exposure to the idelalisib/entospletinib combination therapy was 10 weeks, resulting in respective overall response rates of 60% in the CLL cohort, 36% in the follicular lymphoma cohort, and 17% in patients with DLBCL. (Additional results are summarized in supplemental Table 3.)
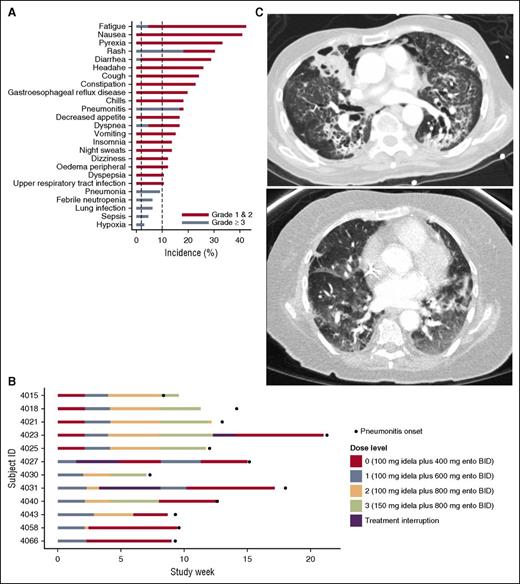
Treatment-emergent diarrhea, rash, and hepatic transaminase elevation were observed in 29%, 30%, and 23% of patients, respectively (Figure 1A) and were generally reversible with treatment discontinuation. However, the study was terminated because of treatment-related pneumonitis, which occurred in 12 of 66 patients (18%), including 11 patients with grade ≥3 pneumonitis. Mean (± standard deviation) time to onset of pneumonitis among the 12 patients was 12 (±4) weeks (Figure 1B) with symptoms characterized by acute-onset dyspnea, cough, hypoxia, and bilateral ground-glass infiltrates on computed tomography scan (Figure 1C), often accompanied by fever and chills. Five patients required ventilator support. With combination treatment discontinuation and supportive care measures (supplemental oxygen and systemic steroids), 7 of 12 patients recovered. Three patients withdrew consent, and further data were not collected. Two of the 12 patients who developed pneumonitis ultimately died of progressive pulmonary dysfunction. No statistically significant association between pneumonitis and prior chemotherapy, prior radiation, or baseline comorbidities was observed; however, this observation is limited by the small sample size. The dose-relatedness is unclear because of the rapid intrapatient dose escalation used in the study design and the delayed occurrence of pneumonitis. Based on our results, a safe dose of the combination cannot be recommended.
Treatment-emergent AEs (N = 66). (A) Serious treatment-emergent AEs included pyrexia, diarrhea, pneumonitis, dyspnea, pneumonia, febrile neutropenia, lung infection, sepsis, and hypoxia. Grade ≥3 events included fatigue, rash, diarrhea, pneumonitis, dyspnea, pneumonia, febrile neutropenia, lung infection, sepsis, and hypoxia. Time to onset of pneumonitis in each patient. (B) The onset of pneumonitis occurred after study day 50 in each patient. In 9 of the 12 patients, pneumonitis onset occurred between days 50 and 100. In 3 of the 12 patients, onset occurred between days 100 and 150. There was no relationship between dose level and pneumonitis onset. Computed tomography images of severe pneumonitis. (C) Representative computed tomography images from 2 patients who developed severe pneumonitis. Diffuse ground-glass opacities and interstitial infiltrates are demonstrated.
Treatment-emergent AEs (N = 66). (A) Serious treatment-emergent AEs included pyrexia, diarrhea, pneumonitis, dyspnea, pneumonia, febrile neutropenia, lung infection, sepsis, and hypoxia. Grade ≥3 events included fatigue, rash, diarrhea, pneumonitis, dyspnea, pneumonia, febrile neutropenia, lung infection, sepsis, and hypoxia. Time to onset of pneumonitis in each patient. (B) The onset of pneumonitis occurred after study day 50 in each patient. In 9 of the 12 patients, pneumonitis onset occurred between days 50 and 100. In 3 of the 12 patients, onset occurred between days 100 and 150. There was no relationship between dose level and pneumonitis onset. Computed tomography images of severe pneumonitis. (C) Representative computed tomography images from 2 patients who developed severe pneumonitis. Diffuse ground-glass opacities and interstitial infiltrates are demonstrated.
In previous investigations, noninfectious pneumonitis has been observed in 2% to 4% of patients treated with PI3K inhibitors.7,8 These events are reminiscent of inhibition downstream of PI3K, with agents targeting mammalian target of rapamycin (mTOR), where pneumonitis has been reported in 2% to 39% of patients.9 The increased severity of pneumonitis in this study may be related to potent inhibition downstream. Burke and colleagues demonstrated a significantly greater decrease in S6 phosphorylation with the combination of idelalisib and entospletinib compared with either agent alone, consistent with increased mTOR inhibition.3 Moreover, pneumonitis has been observed during the development of combined PI3K/mTOR inhibitors, including more severe grade 3 events, despite relatively short durations of administration.10-12
Noninfectious pneumonitis is an immunologic disorder characterized by a T helper 1 (Th1)–type response.13 Interferon-γ is reported to be a central mediator of pneumonitis, and 2 independent reports have demonstrated a significant correlation with serum IL-6 and pneumonitis in patients receiving pulmonary radiation.14-16 Although the pathogenesis of PI3K- and mTOR-inhibitor–related pneumonitis has yet to be elucidated, pleiotropic effects on cytokine production have been demonstrated, consistent with a proinflammatory response.17,18 Further, bronchoalveolar lavage findings of a predominance of CD8+ T cells may suggest a T-cell–mediated autoimmune response.19,20
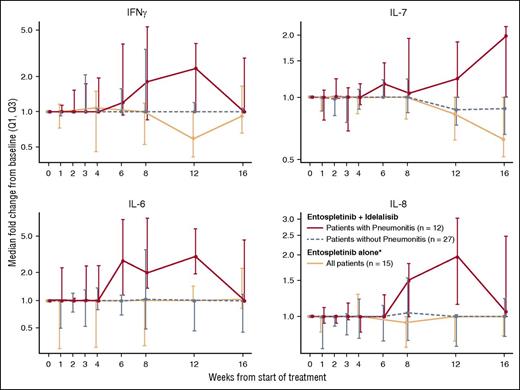
To further investigate the pathogenesis of pneumonitis in our study, we evaluated patient blood samples for changes in circulating cytokines. Supporting the reported immunologic mechanism, a greater median increase in cytokines/chemokines related to immune cell recruitment and Th1-type responses were observed in serum samples of patients developing pneumonitis (n = 12) compared with patients without pneumonitis (n = 27) (Figure 2). Consistent with the delayed occurrence of pneumonitis in this study, the most pronounced cytokine differences occurred by week 12. In contrast, such elevations did not occur in patients in an entospletinib monotherapy study, in which a decline was observed in IL-7 after 8 weeks of treatment (n = 15).21 This suggests that isolated SYK inhibition may not lead to similar inflammatory effects, again consistent with clinical observations.
Cytokine changes over time in patients who did and did not develop pneumonitis. IL-6, IL-7, IL-8, and interferon-γ serum levels were measured by Luminex immunoassays and are depicted separately for patients with (n = 12) and without pneumonitis (n = 27) onset while on idelalisib and entospletinib and for patients treated with entospletinib monotherapy (n = 15). Data are represented as fold change from baseline ×100. Greater elevations of cytokines/chemokines associated with immune cell activation support immunologic mechanisms for the observed pneumonitis pathogenesis. Cell recruitment and Th1 responses, as well as a greater median increase in cytokines/chemokines associated with pneumonitis, were observed in patients with pneumonitis onset compared with patients without pneumonitis onset. *Patients treated on the open-label phase 2 trial of entospletinib.21
Cytokine changes over time in patients who did and did not develop pneumonitis. IL-6, IL-7, IL-8, and interferon-γ serum levels were measured by Luminex immunoassays and are depicted separately for patients with (n = 12) and without pneumonitis (n = 27) onset while on idelalisib and entospletinib and for patients treated with entospletinib monotherapy (n = 15). Data are represented as fold change from baseline ×100. Greater elevations of cytokines/chemokines associated with immune cell activation support immunologic mechanisms for the observed pneumonitis pathogenesis. Cell recruitment and Th1 responses, as well as a greater median increase in cytokines/chemokines associated with pneumonitis, were observed in patients with pneumonitis onset compared with patients without pneumonitis onset. *Patients treated on the open-label phase 2 trial of entospletinib.21
Given this experience and the increasing number of novel agents in development, the current investigation is a cautionary reminder that combinatorial investigations with small-molecule inhibitors need to be initially focused on safety. The study design used a short dose-limiting toxicity window. Nonetheless, the traditional monitoring period for dose-limiting toxicities would have been insufficient given the relatively late occurrence of pneumonitis. As such, we suggest that similar future studies enroll patients in a more conservative manner, without dose escalating individual patients, and include prolonged monitoring given the possibility of unanticipated, as well as late, side effects. Additionally, given the overlapping nature of pathways downstream of the BCR, downstream pathway inhibition may be achieved with lower doses when targeting molecules simultaneously.
In conclusion, BCR inhibition remains a promising therapeutic strategy in lymphoid malignancies. In light of our experience, development of future combinations with agents targeting molecules within the BCR signaling pathways needs to proceed cautiously and with conservative, novel study designs.
Presented in part at the 2014 American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 2, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Impact Communication Partners Inc. for editorial assistance in preparing the manuscript.
This work was supported by Gilead Sciences Inc. P.M.B. is a Lymphoma Research Foundation Clinical Investigator. S.E.S. receives support from the Leukemia Lymphoma Society and SWOG/Hope Foundation.
Authorship
Contribution: P.M.B., S.M.O., S.E.S., L.K.D., and J.D.P. designed and performed research, analyzed data, and wrote the manuscript; B.D.C., G.B.S., D.R.G., A.K.D.L., E.A.-D., A.R., and J.W.F. performed research, analyzed data, and wrote the manuscript; H.L. and J. He performed research and wrote the manuscript; R.E.M. and M.J.H. designed and performed research and wrote the manuscript; and A.J. and J. Hu analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: P.M.B. received consulting fees from Gilead Sciences Inc., Pharmacyclics, AbbVie, Celgene, and Genentech and research funding from Pharmacyclics. G.B.S. received consulting fees from Celgene, Onyx, BioTheranostics, Merck, Millennium, and Bristol-Myers Squibb. S.E.S. received consulting fees from Gilead Sciences Inc., Pharmacylics, Genentech, and Bristol-Myers Squibb and research funding from Gilead Sciences Inc., Janssen, Bristol-Myers Squibb, and Acerta Pharma. B.D.C. received consulting fees from Gilead Sciences Inc., Pharmacyclics, Celgene, and Astellas and research funding from Pharmacyclics, Teva, Celgene, Abbott, Gilead Sciences Inc., and Acerta Pharma. D.R.G. received consulting fees from Celgene, Pharmacyclics, and Genentech. S.M.O. received honoraria from Celgene, Janssen, ProNAi, Pharmacyclics, Regeneron, Gilead Sciences Inc., and Pfizer; consulting fees from CLL Global Research Foundation, Amgen, and Celgene; and research funding from Acerta Pharma, Regeneron, and Gilead Sciences Inc. A.K.D.L. received research funding from Bayer, AstraZeneca, Novartis, Millennium, Gilead Sciences Inc., Minerva, Immunomedics, Incyte Corporation, and OncoMed and consulting fees from Amgen. R.E.M. received honoraria from Celgene and consulting fees from Celgene. A.J. owns stock in Gilead Sciences Inc. E.A.-D. received consulting fees and owns stock in Gilead Sciences Inc. M.J.H. owns stock in Gilead Sciences Inc. A.R. owns stock, patents, royalties, and other intellectual property in Gilead Sciences Inc. J.D.P., H.L., J. He, J. Hu, and A.K.D.L. own stock in Gilead Sciences Inc. J.W.F. received consulting fees from Bayer; research funding from Seattle Genetics, Genentech, Millennium, and Janssen Pharmaceuticals; and honoraria from Bayer and Eisai.
Correspondence: Paul M. Barr, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: paul_barr@urmc.rochester.edu.