Key Points
The ROCK2 inhibitor, KD025, decreases chronic GVHD pathology in multiple murine models.
KD025 inhibits STAT3 phosphorylation to decrease RORγt and Bcl6 expression in both murine and human cells.
Abstract
Chronic graft-versus-host disease (cGVHD) remains a major complication following allogeneic bone marrow transplantation (BMT). The discovery of novel therapeutics is dependent on assessment in preclinical murine models of cGVHD. Rho-associated kinase 2 (ROCK2) recently was shown to be implicated in regulation of interleukin-21 (IL-21) and IL-17 secretion in mice and humans. Here, we report that the selective ROCK2 inhibitor KD025 effectively ameliorates cGVHD in multiple models: a full major histocompatibility complex (MHC) mismatch model of multiorgan system cGVHD with bronchiolitis obliterans syndrome and a minor MHC mismatch model of sclerodermatous GVHD. Treatment with KD025 resulted in normalization of pathogenic pulmonary function, which correlates with a marked reduction of antibody and collagen deposition in the lungs of treated mice to levels comparable to non-cGVHD controls. Spleens of mice treated with KD025 had decreased frequency of T follicular helper cells and increased frequency of T follicular regulatory cells, accompanied by a reduction in signal transducer and activator of transcription 3 (STAT3) and concurrent increase in STAT5 phosphorylation. The critical role of STAT3 in this cGVHD model was confirmed by data showing that mice transplanted with inducible STAT3-deficient T cells had pulmonary function comparable to the healthy negative controls. The therapeutic potential of targeted ROCK2 inhibition in the clinic was solidified further by human data demonstrating the KD025 inhibits the secretion of IL-21, IL-17, and interferon γ along with decreasing phosphorylated STAT3 and reduced protein expression of interferon regulatory factor 4 and B-cell lymphoma 6 (BCL6) in human peripheral blood mononuclear cells purified from active cGVHD patients. Together these data highlight the potential of targeted ROCK2 inhibition for clinical cGVHD therapy.
Introduction
Success of allogeneic hematopoietic stem cell transplantation has been hampered by the prevalence of chronic graft-versus-host disease (cGVHD).1 During cGVHD, donor T cells stimulate donor bone marrow–derived B cells to produce pathogenic antibodies that lead to increased fibrosis in the lung, liver, and skin.2 It is estimated that >50% of allogeneic hematopoietic stem cell transplant recipients develop cGVHD.3 Currently, few therapeutic interventions exist for steroid-refractory cGVHD, and there is an unmet need to develop novel therapeutics. The emerging work suggests that murine models, which individually can recreate certain spectra of tissue pathology and immunopathology associated with GVHD, can be used to test efficacy and decipher the mechanisms of novel pharmacologic agents.4,5
Previous reports demonstrate the importance of germinal center (GC) maintenance by T follicular helper (TFH) cells.4,6,7 This specialized T-cell subset expresses the transcriptional regulator B-cell lymphoma 6 (Bcl6), which is negatively regulated by signal transducer and activator of transcription 5 (Stat5) activation.8,9 TFH cells localize to the GC through the expression of the chemokine receptor CXCR5 and produce interleukin-21 (IL-21).10,11 In the GC, they provide survival signaling to GC B cells and promote the production of pathogenic antibodies. We have previously demonstrated that STAT3 signaling and IL-21 production by pathogenic effector T cells is necessary for establishment of cGVHD.7,12 Our data also suggested that blocking IL-21 signaling could be used as a potential therapeutic in the future.7 More recently, the role of T follicular regulatory cells (TFR) has been described in the development of germinal centers and during autoimmunity.13,14 This subset of regulatory cells coexpresses FoxP3 and Bcl6 and localizes to the GC because of expression of CXCR5; however, these cells negatively regulate the GC response. The GC requires a balance of activating and regulatory cells to allow for production of antibodies and the regulation of autoimmunity.
Although GCs are necessary for cGVHD, the complete role of T cells during cGVHD is still unclear. Other subsets of CD4+ T cells have demonstrated to be important for disease, including TH17 cells.15-17 The TH17 subset has an important role in fighting extracellular pathogens and plays a major role in autoimmunity.18 Previously, it has been demonstrated that T cells lacking the ability to express RORγ are unable to induce GVHD, suggesting the importance of IL-17 for the development of GVHD.12,19 In addition the importance of TH17 cells for the induction of cGVHD have been demonstrated in multiple models of cGVHD.15 Thus, TH17 cells play a critical role in the development of cGVHD and targeting IL-17–producing T cells during cGVHD could lead to novel therapeutics for the disease.
KD025 is an orally available specific Rho-associated kinase 2 (ROCK2) inhibitor. Previous findings demonstrated that inhibition of ROCK2 in healthy patients decreases IL-17 and IL-21 production.20 ROCK2 inhibition by KD025 also shifted the balance of effector cells to T regulatory cells.20 These findings are supported by demonstration of the importance of ROCK2 during autoimmune diseases such as systemic lupus erythematous.21 Furthermore, ROCK2 has been demonstrated to be important in the development of pulmonary fibrosis22 through preventing fibroblast organization and cytokine modulation. We sought to determine if delivery of KD025 could therapeutically reverse established cGVHD in a murine multiorgan system cGVHD model with bronchiolitis obliterans syndrome (BOS) and in a murine model of scleroderma (Scl). In addition we examined the ability for KD025 to inhibit the production of IL-17 and IL-21 in human cGVHD peripheral blood mononuclear cells (PBMCs). Herein we demonstrate that therapeutic treatment of mice with KD025 prevents the development of both BOS and Scl manifestations of cGVHD. KD025 decreased activation of key TH17 and TFH molecules including Stat3, RORγ, and Bcl6 while increasing the activation of Stat5, a negative regulator of TFH cells and positive stimulator of TFR cells. These data combined give strong evidence that KD025 is a potential novel therapeutic for the treatment of cGVHD in the clinic.
Materials and methods
Mice
C57BL/6 (B6; H2b) mice were purchased from the National Cancer Institute. B10.BR (H2k) mice were purchased from Jackson Laboratories. BALB/c, B10.D2-Thy1.2, and B10.D2-Thy1.1 mice were propagated in the animal facility at the Johns Hopkins University Cancer Research Building I. The CD4-cre × Stat3fl/fl mice were generously provided by John O’Shea (National Institutes of Health).23 Mice were housed in a specific pathogen-free facility and used with the approval of the each institution’s animal care committee.
BMT
For BOS model: B10.BR recipients were transplanted as previously described.4,6 Where indicated, cGVHD recipients were given KD025 (30, 100, or 150 mg/kg per animal daily intraperitoneally [IP] or orally [PO]) or 0.4% methylcellulose vehicle from days 28 to 56. KD025 was kindly provided by Kadmon Pharmaceuticals. For the scl model, BALB/c (H2d) mice were given a single lethal irradiation dose of 7.75 Gy by a 137Cs irradiator. Mice were given 10 × 106 B10.D2-Thy1.2 TCD (H2d) BM cells alone or supplemented with 1.8 × 106 CD4+ and 0.9 × 106 CD8+ B10.D2-Thy1.1 T cells purified from the spleen. T cells were obtained using T-cell isolation kits (Dynabeads; Invitrogen). Skin scores and GVHD scores were assessed as previously described.24
Patient samples activation and analysis
Samples were obtained from patients following written informed consent in accordance with the Declaration of Helsinki. Eight viable frozen peripheral blood samples from patient with active clinical manifestations of cGVHD were randomly selected from cell bank for the study that was approved by the institutional review board at the Dana-Farber Cancer Institute.
For analysis of the effects of in vitro ROCK2 inhibition, cryopreserved PBMCs from cGVHD patients were thawed and allowed to rest overnight. Patient PBMCs were incubated with the selective ROCK2 inhibitor, KD025 (2.5 or 10 μM), or dimethyl sulfoxide (DMSO) control (0.1%) on anti-CD3 monoclonal antibody (mAb) (5 μg/mL) and anti-CD28 mAb (5 μg/mL) (eBioscience, San Diego, CA) precoated 24-well plates supplemented with IL-1β (50 ng/mL) and transforming growth factor (TGF)-β (5 ng/mL) (R&D Systems, Minneapolis, MN). Cytokine secretion was determined by enzyme-linked immunosorbent assay after 48 hours using the Human interferon (IFN)-γ Cytoset (Biosource, Camarillo, CA), IL-17 (R&D Systems), and IL-21 (eBioscience). In addition, after 48 hours, the percentage of CD4+CXCR5+PD1+ (TFH) cells was determined by flow cytometry in a guava easyCyte Flow Cytometer machine (EMD Millipore, Billerica, MA).
Statistics
Data were analyzed by a 2-tailed Student t test or 1-way analysis of variance, where indicated, by using the GraphPad Prism software.
Results
KD025 decreases clinical signs of murine multiorgan system cGVHD with BOS through inhibition of Stat3 and activation of Stat5
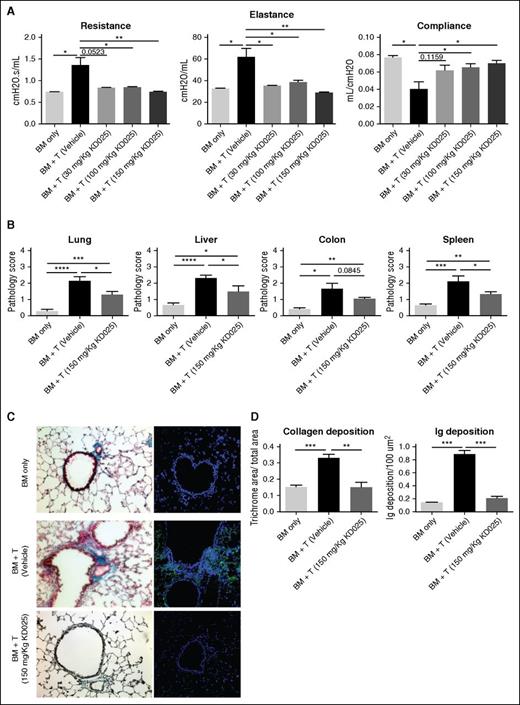
To assess the efficacy of KD025 as a therapeutic intervention during murine cGVHD, we first used a model of BOS that is induced by a full MHC mismatch BMT, where mice develop multiorgan fibrosis associated with increased activation of B cells in the GC and immunoglobulin deposition in cGVHD target organs. Mice were treated with varying doses of KD025 after the establishment of cGVHD on day 28 and analyzed for lung and tissue pathology on day 60. Survival out to day 60 is >80% of animals with minimal weight loss in both cGVHD control mice and mice treated with KD025. Mice treated with KD025 had improvements in their pulmonary function as demonstrated by a decrease in resistance and elastance, with an increase in compliance (Figure 1A). Dose-response data correlate with KD025 serum concentrations (supplemental Figure 1, available on the Blood Web site). Although improvement was seen at all doses, the highest dose of 150 mg/kg KD025 had the largest effect and was used throughout the rest of the experiments. Pathology of the lung, liver, colon, and spleen was evaluated to determine the effects on other cGVHD target organs. Mice treated with the 150-mg/kg dose of KD025 had a significant decrease in the cGVHD pathology in the lung, liver, and spleen compared with the vehicle-treated animals, but there was no significant change in the pathology of the colon (Figure 1B). However, the reversal of pathology was not complete, as KD025-treated mice have significantly more pathologic changes compared with BM-only non-cGVHD control mice.
Therapeutic administration of KD025 decreases cGVHD pathology. B10.BR mice transplanted with B6 BM and T cells were treated with varying doses of KD025 or 0.4% methylcellulose from days 28 to 56. (A) Day 60 pulmonary function tests. (B) Pathology scores for lung, liver, colon, and spleen on day 60 for mice treated with 150 mg/kg KD025. (C) Representative images of Masson’s Trichrome stain (left) and total mouse immunoglobulin (right) in the lungs of mice on day 60 and (D) quantifications. *P < .05; **P < .01; ***P < .001; ****P < .0001. Error bars represent standard error of the mean (SEM); data from 4 separate experiments with n = 8 per group.
Therapeutic administration of KD025 decreases cGVHD pathology. B10.BR mice transplanted with B6 BM and T cells were treated with varying doses of KD025 or 0.4% methylcellulose from days 28 to 56. (A) Day 60 pulmonary function tests. (B) Pathology scores for lung, liver, colon, and spleen on day 60 for mice treated with 150 mg/kg KD025. (C) Representative images of Masson’s Trichrome stain (left) and total mouse immunoglobulin (right) in the lungs of mice on day 60 and (D) quantifications. *P < .05; **P < .01; ***P < .001; ****P < .0001. Error bars represent standard error of the mean (SEM); data from 4 separate experiments with n = 8 per group.
Manifestations of cGVHD in the murine multiorgan system disease and BOS model are highlighted by increased fibrosis in the lungs caused by pathogenic antibody deposition.6 We analyzed lungs from mice for increased collagen in the tissues by trichrome staining. Mice treated with 150 mg/kg had a decrease in the accumulation of collagen in the lungs around the bronchioles compared with mice treated with vehicle (Figure 1C), which correlated with reduced immunoglobulin deposited in the tissues (Figure 1D). These data demonstrate the prevention of pulmonary fibrosis in cGVHD in mice treated with KD025. Treatment with KD025 also preserved the graft-versus-leukemia effect and immune response to viral pathogens (supplemental Figures 2 and 3).
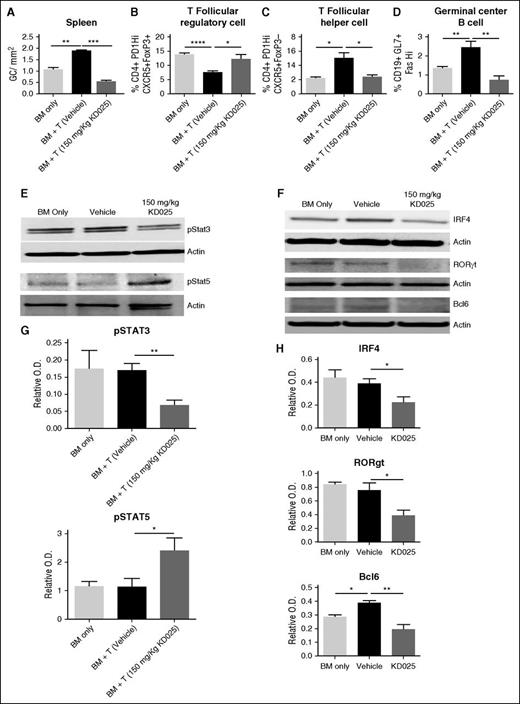
To determine the cellular effects of targeted ROCK2 inhibition during cGVHD, we analyzed spleens of mice on day 60 for the frequency of GCs in situ, TFR cells, TFH cells, and GC B cells (Figure 2A-D). Previously we demonstrated an increase in TFH cells and GC B cells during murine cGVHD in the BOS model.4-7 Mice treated with 150 mg/kg of KD025 had a significant decrease in the frequency of GCs in their spleens on day 60 (Figure 2A) Furthermore, mice treated with KD025 had a significant increase in the frequency of TFR cells (Figure 2B; supplemental Figure 4) and a decrease in the presence of TFH cells (Figure 2C; supplemental Figure 4). In addition, KD025 was able to decrease both GC B and TFH cells in an immune response in the absence of transplantation (supplemental Figure 5). The combination of decreased TFH cells and increased TFR cells potentially leads to the decrease in GC B cells present in the spleens of mice (Figure 2D; supplemental Figure 4). These data support that KD025 is effective at decreasing the immunopathology associated with cGVHD that correlates with KD025-mediated inhibition of TFH supported GC formation and production of antibody-secreting cells.
KD025 inhibits Stat3 phosphorylation and increases Stat5 activation decreasing the germinal center response. Day 60 spleens from B10.BR mice transplanted with B6 BM and T cells were analyzed for the (A) frequency of GCs in situ, (B) T follicular regulatory cell frequency, (C) T follicular helper cell frequency, and (D) germinal center B cell frequency. (E) Western blots of whole splenocytes for phosphorylation of Stat3 or Stat5 normalized to actin and (F) IRF4, RORγt, and Bcl6, which are quantified from 5 blots from 2 independent experiments in G and H, respectively. *P < .05; **P < .01; ***P < .001; ****P < .0001. Error bars represent SEM; data from 3 separate experiments with n = 8 per group.
KD025 inhibits Stat3 phosphorylation and increases Stat5 activation decreasing the germinal center response. Day 60 spleens from B10.BR mice transplanted with B6 BM and T cells were analyzed for the (A) frequency of GCs in situ, (B) T follicular regulatory cell frequency, (C) T follicular helper cell frequency, and (D) germinal center B cell frequency. (E) Western blots of whole splenocytes for phosphorylation of Stat3 or Stat5 normalized to actin and (F) IRF4, RORγt, and Bcl6, which are quantified from 5 blots from 2 independent experiments in G and H, respectively. *P < .05; **P < .01; ***P < .001; ****P < .0001. Error bars represent SEM; data from 3 separate experiments with n = 8 per group.
Targeted ROCK2 inhibition in healthy human patients simultaneously downregulates STAT3 and upregulates STAT5 phosphorylation.20 To determine the molecular mechanism of immunologic effects of KD025 during cGVHD, spleens harvested from animals on day 60 were analyzed for the expression of phosphorylated Stat3 and Stat5. Mice treated with 150 mg/kg KD025 have a robust decrease in levels of Stat3 phosphorylation (Figure 2E and quantified in 2G), whereas Stat5 phosphorylation was increased (Figure 2E and quantified in 2G). In addition to changes in Stat signaling, mice treated with KD025 had a dose-dependent decrease in expression of key TH17 proteins interferon regulatory factor 4 (IFR4) and RORγτ, as well as decreased expression of the TFH master transcriptional regulator Bcl6 (Figure 2F and quantified in 2H).
Stat3 expression is required in donor T cells for the development of cGVHD
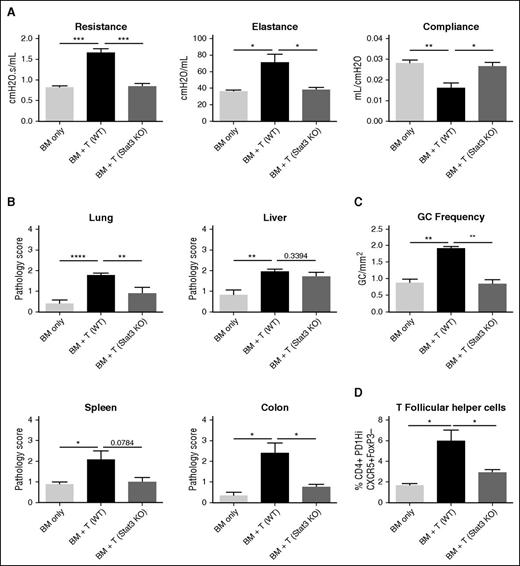
Stat3 signaling in CD4+ T cells has been demonstrated to be important in the induction of acute GVHD and is important in the development of Scl cGVHD.12,25 However, the role of Stat3 signaling in CD4+ T cells has yet to be fully explored in a B cell–dependent, multiorgan system cGVHD model. To determine the role of Stat3 in the induction of cGVHD, we transplanted B10.BR mice with wild-type (WT) B6 BM and either T cells from WT T cells or CD4-cre × Stat3fl/fl (Stat3 knockout [KO]) donor mice that have a deletion of Stat3 in all mature T cells. Mice given Stat3-deficient T cells did not develop pathogenic pulmonary function, as mice did not have increased resistance and elastance or decreased compliance and had lung function similar to healthy non-cGVHD controls (Figure 3A). We also analyzed pathologic changes in mice when transplanted with Stat3 KO T cells (Figure 3B). Mice had a significant decrease in the pathology of the lung and colon. However, there was no statistically significant difference in the spleen, even though pathology was substantially decreased. Liver injury appeared unaffected (Figure 3B). Mice transplanted with WT BM and with Stat3 KO T cells had a decrease in the frequency of the GC reactions present and TFH cells in the spleen on day 60 (Figure 3C-D). These therapeutic effects are also present when a Stat3 inhibitor is used as demonstrated by pulmonary function tests (supplemental Figure 6). These data demonstrate the critical role of Stat3 in T cells to establish GC reactions and clinical pathology associated with cGVHD in a multiorgan system cGVHD model with BOS.
Stat3 expression in T cells is necessary for the development of cGVHD. B10.BR mice were transplanted with B6 WT BM and either WT or Stat3-deficient T cells. (A) Day 60 pulmonary function tests. (B) Pathology scores from lung, liver, spleen, and colon on mice killed on day 60. (C) Frequency of germinal centers in spleens of mice killed on day 60. (D) Frequency of T follicular helper cells on day 60. *P < .05; **P < .01; ***P < .001; ****P < .0001. Error bars represent SEM; data from 2 separate experiments with n = 8 per group.
Stat3 expression in T cells is necessary for the development of cGVHD. B10.BR mice were transplanted with B6 WT BM and either WT or Stat3-deficient T cells. (A) Day 60 pulmonary function tests. (B) Pathology scores from lung, liver, spleen, and colon on mice killed on day 60. (C) Frequency of germinal centers in spleens of mice killed on day 60. (D) Frequency of T follicular helper cells on day 60. *P < .05; **P < .01; ***P < .001; ****P < .0001. Error bars represent SEM; data from 2 separate experiments with n = 8 per group.
KD025 treatment decreases clinical scores in sclerodermatous chronic GVHD
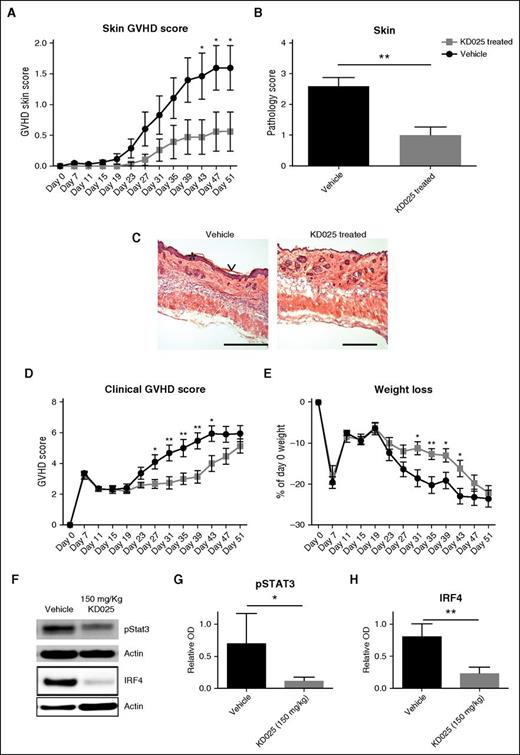
To verify the therapeutic benefit of KD025 in other manifestations of cGVHD, we used a minor-mismatch model of Scl cGVHD. In this model, BALB/c mice were transplanted with B10.D2 bone marrow and T cells and treated with 150 mg/kg KD025 starting on day 19 when mice started to develop clinical signs of the disease. Treatment with KD025 significantly decreased the skin clinical cGVHD most evident after about 3 weeks of therapy and skin pathology scores compared with the vehicle-treated animals (Figure 4A-B). Epidermal hyperplasia, keratosis, infiltration of nucleated cells into the dermis, and destruction of hair follicles were noted in mice given vehicle compared with mice treated with KD025 (Figure 4C). The skin pathology correlates with a decrease in clinical scores and weight loss seen from ∼3 to 6 weeks after transplantation in mice treated with KD025 compared with the vehicle-treated mice (Figure 4D-E). The improvement in clinical scores was complemented by a reduction in pSTAT3 and IRF4 expression in splenocytes of KD025-treated mice consistent with previously presented data (Figure 2), demonstrating the critical role of STAT3 in pathogenesis of this model (Figure 4F and quantified in 4G-H). Thus, targeted ROCK2 inhibition decreases the total pathology and skin disease seen in a scl model of cGVHD via a STAT3-dependent mechanism.
Reversal of clinical signs of sclerodermatous cGVHD with KD025 treatment. BALB/c mice were transplanted with B10.D2 BM and T cells and given either 150 mg/kg KD025 or 0.4% methylcellulose vehicle control starting after initial signs of cGVHD and harvested on day 51 following transplantation. (A) Clinical skin scores, (B) skin pathology, (C) highlights of pathology (*epidermal hyperplasia and ∨keratosis; bar, 500 μM), (D) clinical GVHD scores, and (E) percent weight loss. Whole spleens were assessed for Stat3 phosphorylation normalized to actin and expression of IRF4 (F and quantified in G and H, respectively). *P < .05; **P < .01. Error bars represent SEM; data from 2 separate experiments with n = 8 per group.
Reversal of clinical signs of sclerodermatous cGVHD with KD025 treatment. BALB/c mice were transplanted with B10.D2 BM and T cells and given either 150 mg/kg KD025 or 0.4% methylcellulose vehicle control starting after initial signs of cGVHD and harvested on day 51 following transplantation. (A) Clinical skin scores, (B) skin pathology, (C) highlights of pathology (*epidermal hyperplasia and ∨keratosis; bar, 500 μM), (D) clinical GVHD scores, and (E) percent weight loss. Whole spleens were assessed for Stat3 phosphorylation normalized to actin and expression of IRF4 (F and quantified in G and H, respectively). *P < .05; **P < .01. Error bars represent SEM; data from 2 separate experiments with n = 8 per group.
KD025 inhibits production of IL-21, IL-17, and IFNγ in human cGVHD PBMCs
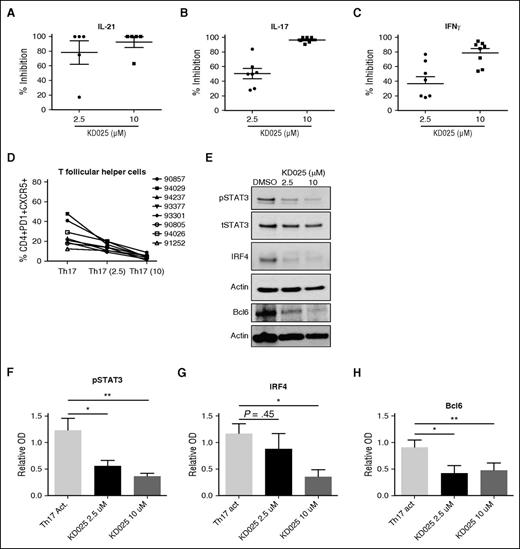
To further validate the therapeutic potential of ROCK2 inhibition for cGVHD, we tested the effects of KD025 on cytokine secretion in ex vivo–stimulated PBMCs obtained from active cGVHD patients. Total PBMCs were cultured in Th-17 skewing media containing 2.5 or 10 μM of KD025 or DMSO, and levels of IL-21, IL-17, and IFNγ were determined after 48 hours (Figure 5A). KD025 inhibited ∼80% of IL-21 production in the lower concentration and >90% in the higher concentration compared with DMSO-treated cells. Inhibition of IL-17 was increased from ∼50% inhibition at the lower concentration to nearly 100% inhibition at the higher concentration. Finally, production of IFNγ was decreased ∼40% at a lower concentration of KD025 to ∼80% in the higher concentration compared with DMSO controls. These findings suggest that similarly to healthy controls, ROCK2 inhibition potently downregulates proinflammatory cytokine secretion in PBMCs from active cGVHD patients.20 Importantly, the concentration of KD025 used for ex vivo cultures of cGVHD-derived PBMCs, as well as detected in plasma of treated cGVHD mice (supplemental Figure 1), was comparable to levels observed in healthy human subjects during oral administration of the drug in the phase 1 clinical trial.20 Moreover, the presence of KD025 leads to a dose-dependent decrease in the percentage of human CD4+CXCR5+PD1+ cells (TFH cell subset) induced by Th17-skewing of cGVHD-derived PBMCs (Figure 5D). These cells were also analyzed for phosphorylated Stat3 and the expression of IRF4 and Bcl6. When human cells were treated with increasing doses of KD025, there was a decrease in the phosphorylation of Stat3 (Figure 5E-F). This coincided with a decrease in the expression of both IRF4 and Bcl6 (Figure 5E and quantified in 5G-H). These data support that targeted ROCK2 inhibition downregulates the production of key cGVHD cytokines and prevents the conversion of T cells to TFH cells that are necessary for the progression of disease.
Immunologic effects of KD025 on human cGVHD. PBMCs from patients with active cGVHD were analyzed for their ability to produce (A) IL-21, (B) IL-17, and (C) IFNγ when treated with 2.5 μM, 10 μM, or DMSO vehicle for 48 hours. (D) The frequency of T follicular helper cells present and (E) expression of pStat3 normalized to actin, IRF4, and Bcl6 in samples treated with KD025, quantified in F, G, and H, respectively. *P < .05; **P < .01. Error bars represent standard deviation.
Immunologic effects of KD025 on human cGVHD. PBMCs from patients with active cGVHD were analyzed for their ability to produce (A) IL-21, (B) IL-17, and (C) IFNγ when treated with 2.5 μM, 10 μM, or DMSO vehicle for 48 hours. (D) The frequency of T follicular helper cells present and (E) expression of pStat3 normalized to actin, IRF4, and Bcl6 in samples treated with KD025, quantified in F, G, and H, respectively. *P < .05; **P < .01. Error bars represent standard deviation.
Discussion
ROCK2 is critical in regulating of proinflammatory cytokines such as IL-21 and IL-17 in both mice and humans. Several studies have demonstrated that these cytokines play an instrumental role in the pathogenesis of cGVHD. Here we report that targeted inhibition of ROCK2 by the small molecule inhibitor KD025 is capable of preventing the clinical and immunologic symptoms of cGVHD in 2 pathologically distinct murine models and demonstrated a common mechanism of downregulation of STAT3 phosphorylation (Figures 2-4). Although no models completely mimic all aspects of human cGVHD, for example, this murine model of cGVHD demonstrates peribronchial fibrosis with little endobronchial involvement unlike human disease, which is an endobronchial disease. However, they can be used to study the immune response and reversal of pathology in a model system. KD025 significantly inhibited IL-21, IL-17, and IFNγ secretion and prevented expression of Stat3, IRF4, and Bcl6 in PBMCs from active cGVHD patients (Figure 5). Concurrently, our data also demonstrate the increase in activation of Stat5 with the administration of KD025 in vivo in cGVHD mice (Figure 2E). These signaling events were further verified by a decrease in both TFH cells and an increase in TFR cells in KD025-treated cGVHD mice (Figure 2B-C). Overall, our data demonstrate that KD025 has a major therapeutic effect on key cGVHD cytokines and signaling pathways involved in pathogenesis of the disease in both mice and humans.
It was previously demonstrated that KD025 inhibited Stat3 phosphorylation and increased Stat5 phosphorylation in healthy human samples.20 Stat3 is important for the induction and the progression of disease in cGVHD murine models as demonstrated by mature donor T cells requiring Stat3 for development of disease (Figure 3). Although immunoglobulin deposition has been noted in the liver and strategies that target GC B cells have reduced liver pathology, the pathogenic role of immunoglobulin deposition in liver injury is unclear. Previous data using LTβR-immunoglobulin has demonstrated that even without GCs or immunoglobulin deposition, there can still be liver injury, independent of other tissues.6 The use of a Stat3 inhibitor has demonstrated efficacy in the BOS model (supplemental Figure 6) and in the scl model of cGVHD.12 The in vivo inhibition of Stat3 phosphorylation by KD025 in a murine model of cGVHD leads to a decrease in IRF4 and RORγt expression in splenic cells. IRF4 is necessary for the regulation of IL17 during autoimmunity in mice.26 The expression of RORγ is necessary for conversion of T cells to a TH17 program, which has been demonstrated to be important for cGVHD.15,27 In addition, Zanin-Zhorov et al20 demonstrated that the increase in Stat5 expression skews the T regulatory cell/TH17 balance to favor a more regulatory environment. This inversion is likely important for the treatment of cGVHD by increasing the TFR cell presence and decreasing the pathogenic TFH cells. It is not clear if the decrease in GC B cells (Figure 2D) is due to a direct action of KD025 on B cells or if it is caused by the decrease in TFH cells and increase of TFR cells. Activation of B cells through increased survival factors and B-cell receptor activation has previously been demonstrated to occur during cGVHD.4,28,29 The inhibition of Stat3 signaling in B cells could provide additional support for therapeutic potential of KD025 in cGVHD. Further experiments using cells from mice with a conditional deletion of ROCK2 will be necessary to determine the role of ROCK2 in T and B cells and regulation of the GC reaction. The decrease in pathology is highlighted by the decrease in collagen and immunoglobulin staining in lungs of mice treated with KD025 compared with vehicle-treated mice (Figure 1C-D). With a decrease in GC activity, the pathogenic antibodies necessary to cause tissue damage and fibrosis are removed. Together these data suggest the importance of ROCK2 inhibition in production of pathogenic antibody and development of disease.
Restoring tolerance through increasing T regulatory cell number and function has long been demonstrated to be an important factor in GVHD treatment.30-32 Here we show the increased phosphorylation of Stat5 when mice are treated with KD025 compared with vehicle-treated mice (Figure 2E). Stat5 activation promotes expression of FoxP3 expression and reduces lethal acute GVHD when constitutively active.33 We further describe an increase in TFR cells in mice with ROCK2 inhibition compared with vehicle-treated controls (Figure 2B). TFR cells are important in the regulation of the GC and prevention of autoimmunity.13,14,34 The role of TFR cells in cGVHD is poorly understood. Our results show a significant decrease of TFR cells during active cGVHD compared with healthy non-GVHD transplant control mice (Figure 2B). This suggests that TFR cells either do not receive enough survival signals or are eliminated during cGVHD, leading to propagation of disease. TFR cells could decrease cGVHD pathology by decreasing the GC response and preventing pathogenic antibody production.
The decrease in fibrosis seen in mice treated with KD025 is consistent with the role of ROCK in pulmonary fibrosis. For example, the pan-ROCK inhibitor, fasudil, decreases the development of pulmonary fibrosis in a bleomycin model and can induce the regression of already established fibrosis.22 We demonstrate that fibrosis surrounding the bronchioles in the lung is decreased (Figure 1D) following treatment with KD025 in a murine model where lung fibrosis is highlighted.6 However, it is not clear if fibrosis is decreased by reducing damage to the tissues caused by Ab deposition or if KD025 is decreasing TGFβ production. Previously, TGFβ was found to be produced by monocytes in the skin of cGVHD animals and neutralization with an anti-TGFβ antibody decreased disease.35 Monocytes are essential for pulmonary fibrosis in our cGVHD model.15 Because ROCK2 expression supports TGFβ production,36 KD025 inhibition of ROCK2 expression would result in lower TGFβ production and consequently may directly decrease cGVHD fibrosis. Because KD025 has multiple effects on the immune response as well as fibrosis maintenance and formation, KD025 could have advantages over the use of a STAT3 inhibitor alone in treating cGVHD.
Scleroderma associated with cGVHD is a serious manifestation seen in clinical practice. We used a Scl cGVHD model to examine the ability to prevent clinical disease. Mice treated with KD025 had a delay in the development of disease and decrease in skin GVHD scores (Figure 4). The delay in skin disease was not effective at preventing all manifestations of cGVHD, including weight loss, hunching, and fur loss. In addition, animals were killed late in the experiment due to disease progression (Figure 4D-E). This decrease in scleroderma development was confirmed by the decrease in Stat3 phosphorylation and IRF4 expression (Figure 4F-H). The ability to prevent the development of established disease is an essential quality of effective therapeutics for cGVHD.
In addition to murine models, we are able to replicate many of the KD025 immunologic results using human PBMCs from active cGVHD patients. We demonstrated that KD025 in a dose-dependent manner was able to inhibit the production of IL-21, IL-17, and IFNγ compared with DMSO controls (Figure 5A-C). There is mounting evidence that these 3 cytokines have important roles in cGVHD, transplantation, and autoimmunity.7,37-39 We also showed that KD025 treatment decreases the presence of TFH cells in cGVHD patient samples (Figure 5D). This is supported by a decrease in Bcl6 expression in treated cells (Figure 5E,H). In addition the phosphorylation of Stat3 is significantly decreased along with expression of IRF4 (Figure 5E-G). These data suggest that in human cGVHD PBMCs there is a decrease in TFH cells and TH17 cells, consistent with previous reports.20
Previous reports demonstrated a survival advantage in acute GVHD, which is a proinflammatory setting, when mice were treated with fasudil.40 KD025 is specific to ROCK2, whereas fasudil blocks both isoforms of ROCK. Selective inhibition of ROCK2 could have greater therapeutic index over the inhibition of both isoforms. Recently, Zandi et al41 demonstrated that targeting ROCK2 restores the beneficial macrophage balance during age-related macular degeneration whereas targeting ROCK1 increases pathogenic macrophages polarization. Although inhibition of all ROCK isoforms has therapeutic advantages, the specific inhibition of ROCK2 could have greater effects on the immune regulation with fewer off-target side effects. Moreover, because cGVHD has distinct pathophysiologic mechanisms from acute GVHD,42 the clinical and immunologic effects of KD025 on ameliorating cGVHD in a broad range of clinical manifestations typified by multiorgan system disease with BOS to scleroderma, could not be surmised from the prior acute GVHD study40 that also used a pan-ROCK rather than the more selective ROCK2 inhibitor. Nevertheless, KD025 was in fact effective in cGVHD models. Indeed, the novel regulation of the GC and changes to cytokine production by ROCK2 inhibition with KD025 makes targeting this pathway viable for both the acute and chronic forms of GVHD that can be driven by IL-21 and IL-17 expression and impeded by T regulatory cell mediated suppression.
Together, these data support that ROCK2 inhibition is a potential novel therapeutic for the treatment of cGVHD. KD025 is currently in phase 2 clinical testing and shows promising effects for psoriasis, systemic lupus erythematous, and other autoimmune or fibrotic diseases. The decrease in presence and activity of TFH and TH17 cells along with an increase in TFR cells could establish a restoration in the immune response. Furthermore, ROCK2 inhibition has a profound effect on decreasing lung fibrosis. In summary, our data from preclinical murine models and human data suggest that KD025 should be in consideration of clinical trials alone or with other agents for the treatment of cGVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kadmon Corporation for providing KD025; Drs John J. O’Shea and Yuka Kanno for providing CD4-cre X Stat3fl/fl cells; Dr John Ryan for critical reading of the manuscript; and Randy Donelson for tissue preparation and staining.
This study was funded by the National Institutes of Health, National Cancer Institute grants P01 CA142106-06A1 and 5P01-CA047741-20 (B.R.B.) and R01-CA122779 (L.L.); National Institute of Allergy and Infectious Diseases grants P01 AI 056299, T32 AI 007313, and K08HL107756; Leukemia and Lymphoma Society Translational Research grants 6458-15 and 6462-15; and the Kadmon Corporation.
Authorship
Contribution: R.F. designed experiments, performed experiments, discussed results, and wrote the paper; K.P., J.D., D.K.R., P.A.T., A.V., L.L., M.S.N., J.M.W., W.C., A.T., and E.G.A. designed and performed experiments and edited the paper; A.P.-M. performed histologic analyses, discussed experimental design, and edited the paper; and K.K.P.M., G.R.H., J.S.S., W.J.M., I.M., D.M., J.K., C.S.C., J.H.A., J.R., S.D.W., A.Z.-Z., and B.R.B. designed experiments, discussed results, and edited the paper.
Conflict-of-interest disclosure: M.S.N., J.M.W., W.C., A.T., S.D.W., and A.Z.-Z. are employees of Kadmon Corporation. All other authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, MMC 109, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu; or Alexandra Zanin-Zhorov, Kadmon Research Institute, 450 East 29th St, New York, NY 10016; e-mail: alexandra.zanin-zhorov@kadmon.com.
References
Author notes
A.Z.-Z. and B.R.B. contributed equally to this work.