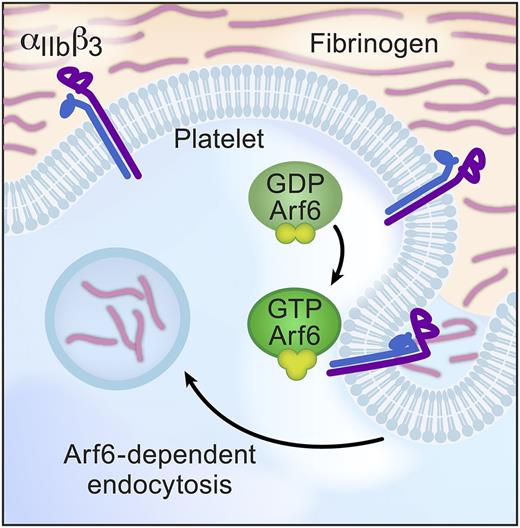
In this issue of Blood, in a departure from studies of classic platelet function, Huang et al turn their attention to endocytosis and show that adenosine 5′-diphosphate–ribosylation factor 6 (Arf6) plays a key role in fibrinogen engulfment.1 Although platelets are known to bind, absorb, and load their granules with plasma proteins, this report is one of the first to explore mechanisms that control endocytosis in this anucleate cell. Huang et al demonstrate that Arf6-dependent endocytosis is restricted to fibrinogen, implying that Arf6 also modulates trafficking of αIIbβ3 integrins in platelets. Consistent with this notion, deletion of Arf6 in platelets enhances spreading on fibrinogen and accelerates clot retraction (see figure). However, activation of surface αIIbβ3 is unaffected, and Arf6 deficiency does not alter thrombosis in vivo. These incongruous results point toward the complexity of anucleate platelets and the need for more detailed studies to understand intracellular trafficking, recycling, and endocytosis in platelets and their precursors.
Model of Arf6-mediated fibrinogen endocytosis. Arf6 is a key intracellular trafficking molecule and, in platelets, enhances fibrinogen engulfment into intracellular vesicles. Arf6 also influences platelet spreading and clot retraction through mechanisms that remain incompletely understood. GDP, guanosine diphosphate; GTP, guanosine triphosphate.
Model of Arf6-mediated fibrinogen endocytosis. Arf6 is a key intracellular trafficking molecule and, in platelets, enhances fibrinogen engulfment into intracellular vesicles. Arf6 also influences platelet spreading and clot retraction through mechanisms that remain incompletely understood. GDP, guanosine diphosphate; GTP, guanosine triphosphate.
Arf6 is a small GTPase that localizes to membranes and endosomal compartments.2 In nucleated cells, Arf6 regulates endocytic membrane trafficking and thereby impacts cell motility, cell division, and lipid homeostasis. Arf6 has also been linked to actin remodeling, which may explain why genetic disruption and pharmacologic inhibition of Arf6 in mouse and human platelets, respectively, affects platelet spreading. Like other small GTPases, Arf6 cycles between an active GTP-bound and inactive GDP-bound conformation. When cycling between these 2 states, Arf6 facilitates ligand internalization at cell surfaces, endosomal recycling, and fusion of endosomal membranes with plasma membranes. Previous studies from Whiteheart’s group3,4 showed that Arf6 rapidly converts from a GTP- to GDP-bound state in activated human platelets and that a second wave of integrin-dependent outside-in signaling suppresses return of Arf6-GDP to Arf6-GTP. Kanamarlapudi et al5 also found that Arf6 regulates P2Y trafficking in platelets. The current work extends both of these observations and provides unequivocal evidence that Arf6 regulates the internalization of fibrinogen in platelets.
By modulating the internalization and trafficking of integrins, Arf6 strongly impacts cell migration, invasion, and shape in nucleated cells. In regard to cell shape, Arf6-deficient platelets spread more readily on fibrinogen compared with controls. The reasons for enhanced spreading are not completely understood and may be due to more rapid recycling of activated αIIbβ3 to and from the surface of Arf6-deficient platelets. However, αIIbβ3 surface levels and inside-out activation of the integrin are similar between Arf6 knockout and wild-type platelets, as is downstream activation of Rac1 (Ras-related C3 botulinum toxin substrate 1) and RhoA (Ras homolog gene family, member A). As hypothesized by the authors, future studies are needed to determine whether Arf6 actually controls αIIbβ3 trafficking in platelets by redirecting this critical integrin and its binding partner (fibrinogen) into “fast-track” endocytic pathways that reduce surface and/or internal residence time. Delineating reasons why Arf6-deficient platelets display accelerated clot retraction but have normal aggregation responses will also be informative.
The study by Huang et al is undoubtedly a prelude to future investigations focused on platelet endocytosis. As one can imagine, alterations in the endocytotic process may greatly affect the repertoire, level, and/or activity of proteins in platelet granules. This may have important implications in human diseases where alterations in the regulation and release of platelet granule proteins may contribute to thrombotic events or bleeding. For example, higher levels of plasma fibrinogen have been associated with an increased risk of cardiovascular disease.6 In other acute infectious settings, such as sepsis, fibrinogen levels may be markedly increased or decreased (especially with disseminated intravascular coagulation), influencing both bleeding and thrombosis.7
Last, this report extends previous studies from the same group demonstrating that the Arf6 pathway is present and active in platelets, albeit the results are somewhat discordant between mouse and humans. In 2006, they showed that Arf6 inhibitors effectively blocked spreading of human platelets on collagen, whereas Arf6-deficient mouse platelets exhibit increased spreading on fibrinogen with no effective change when adherent to collagen. In human platelets, the ARF inhibitors also blocked many of the functional responses and signaling steps that were unaffected in the current study. There are many possible explanations for these differential responses, including off-target effects of the Arf6 inhibitors or differential expression of Arf6 signaling components between mouse and human platelets. Although responses may differ partially between mice and humans, these studies points toward a central role for Arf6 in mediating platelet endocytosis. Perhaps more importantly, the current study highlights the power and limitations of comparing functional responses between mouse and human platelets. This is an important point for investigators to arbitrate as they seek to understand traditional and new functions of platelets and their importance in health and disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.