In this issue of Blood, Shen et al demonstrate that the vesicular stomatitis virus (VSV)–murine interferon β (IFNβ)–sodium iodide symporter (NIS) (VSV-mIFNβ-NIS) oncolytic virus has significant antileukemia activity, which is enhanced when combined with an anti–programmed death–ligand 1 (PD-L1) antibody.1
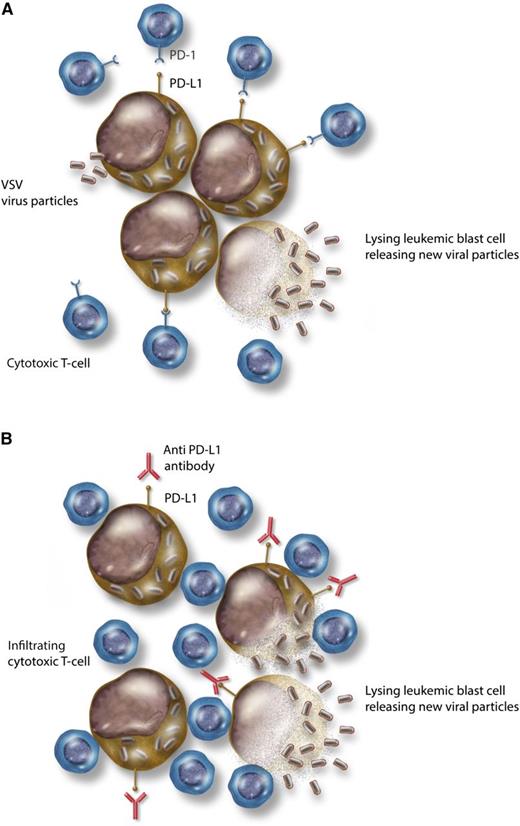
Combining immune checkpoint blockade with an oncolytic virus. (A) VSV infecting leukemic blasts, replicating within them, and causing cell lysis. PD-L1, expressed on leukemic blasts, interacts with PD-1 on cytotoxic T lymphocytes and suppresses their effector function. (B) Addition of anti–PD-L1 antibody disrupts the immune checkpoint interaction and releases the T-cell inhibition. Effector T lymphocytes are now able to infiltrate and potentiate an immune response against the virally infected blasts, increasing cell kill. Professional illustration by Somersault18:24.
Combining immune checkpoint blockade with an oncolytic virus. (A) VSV infecting leukemic blasts, replicating within them, and causing cell lysis. PD-L1, expressed on leukemic blasts, interacts with PD-1 on cytotoxic T lymphocytes and suppresses their effector function. (B) Addition of anti–PD-L1 antibody disrupts the immune checkpoint interaction and releases the T-cell inhibition. Effector T lymphocytes are now able to infiltrate and potentiate an immune response against the virally infected blasts, increasing cell kill. Professional illustration by Somersault18:24.
Approximately 30% to 40% of patients with acute myeloid leukemia (AML) may be refractory to initial therapy, and a majority of patients who achieve a first complete remission will experience relapse.2 Relapsed and refractory AML is characterized by chemotherapy-resistant disease for which newer modalities of therapy are critically needed. Targeted small-molecule inhibitors, monoclonal antibodies, and cellular therapies are currently in active development. Oncolytic virotherapy is an emerging therapeutic modality that uses live, replicating viruses to target, infect, and kill cancer cells in vivo. This strategy of a systemically administered, self-replicating vehicle of treatment in “one shot” is appealing in a systemic disease like AML, where the dividing malignant cells are disseminated both intravascularly and in diverse tissues. The ability of oncolytic viruses to repeatedly infect cancer cells also carries the potential for eradicating minimal residual disease.
Oncolytic viruses are engineered to be nonpathogenic to normal cells by removing virulence factors, while still maintaining their tropism to cancer cells.3 Defects in innate immune signaling within tumors may actually provide an ideal setting for viral infection and replication. Cytotoxicity using oncolytic viruses is thought to occur via several mechanisms, including: (1) direct lysis and cell death after synthesis of new viral particles, and (2) stimulation of the host cytotoxic immune response with direct cell priming and epitope spread after the local cellular lysis.3 Several phase 1/2 clinical trials using oncolytic viruses have been conducted in patients with solid and hematopoietic malignancies, validating this as a viable approach.3 Recently, a phase 3 trial demonstrated improved responses and prolonged overall survival in patients with advanced melanoma treated with an oncolytic, modified herpes simplex virus, talimogene laherparepvec.4
The current study uses an engineered strain of VSV, a single-stranded RNA virus that is minimally pathogenic in humans and has demonstrated oncolytic activity.5 The VSV-mIFNβ-NIS construct is engineered to include a gene expressing IFNβ that enhances tumor cell selectivity, as well as the NIS transgene, which encodes a sodium-iodide symporter and allows noninvasive in vivo imaging. The local expression of IFNβ can also promote local innate cellular immune responses, potentiating antitumor activity. This strain of VSV has been previously used by the authors, demonstrating potent cytotoxicity in a myeloma model.5
The host immune response plays an important role in the surveillance and eradication of cancer. The propagation and spread of malignant cells is often marked by a permissive, immunosuppressive tumor microenvironment that impedes host immune response. Recently, some of these mechanisms of immune evasion have been elucidated. Upregulation of PD-L1 on tumor or associated stromal cells can interact with the programmed death–1 (PD-1) receptor on infiltrating cytotoxic T cells, abrogate their antitumor response, and “keep them at bay.”6 Disrupting this immune checkpoint interaction could reverse tumor-mediated immunosuppression and augment antitumor T-cell responses. Monoclonal antibodies to PD-1 and PD-L1 have been developed to block this interaction and have demonstrated significant, durable clinical responses in patients with solid and hematopoietic malignancies.6 The current study aims to exploit the activity of immune checkpoint inhibitors to potentiate the local host immune response after viral infection and increase antileukemia activity. Viral-mediated lysis with epitope spread could create an “antigen-rich” environment in a disease like AML that is not known to have a large number of neoantigens.7 This, coupled with an activated local immune response, could be a compelling approach.
Using syngeneic, immune-competent mouse models, Shen and colleagues demonstrate selective virus infectivity of tumor cells after systemic administration, dose-dependent antitumor activity, and enhancement of antileukemia activity with the addition of an anti–PD-L1 antibody. In a model of disseminated AML, the virus was effective at reducing AML burden from blood, bone marrow, liver, and spleen after systemic administration, particularly when combined with the anti–PD-L1 antibody. This translated into a significant survival benefit for virus-treated mice compared with controls—an effect that was again enhanced by the addition of the anti–PD-L1 antibody. Depletion of mouse natural killer cells and CD8+ T cells, but not CD4+ T cells, negated the survival benefit, suggesting that these immune cells were important for the antileukemia response. When the mice tissues were examined at necropsy, the authors demonstrated robust VSV recovery from the tumors and high PD-L1 expression in saline-treated controls. Those tumors treated with the anti–PD-L1 antibody showed increased infiltration by CD8+ cytotoxic T cells and decreased PD-L1 expression. Next, the authors report a critical observation to support the concept of crosspriming. From the treated mice, the authors were able to isolate both VSV-specific and leukemia-specific cytotoxic T cells, with the highest levels found in the combination-treated cohort. Together, these observations provide evidence that the viral infection alone and in combination with the anti–PD-L1 antibody is functioning also as an immunotherapeutic approach. Finally, the authors performed ex vivo analysis of primary patient samples and demonstrated high infectivity with VSV among patients with chronic myelomonocytic leukemia and monocytic AML.
This preclinical study outlines a very novel approach to AML treatment, combining an oncolytic virus with immune checkpoint inhibition, revealing virotherapy as an immunotherapy (see figure). Such a strategy could expand the spectrum of malignancies that may be amenable to immunotherapy outside of those that are most immunogenic. These results need to be confirmed in future studies, but several challenges remain. Systemic virus administration in patients with AML would require achievement of large viral titers and overcoming the host serum and immune factors that could neutralize the virus. Clearance and sequestration by the liver and spleen, although not observed in the mouse model, are important obstacles with systemic administration in humans.3 As evidenced in the ex vivo patient samples, the virus robustly infected only a subset of cases. Therefore, choosing the right virus and appropriately targeting it to the malignant leukemia cells will remain an ongoing challenge. Because the immune response appears necessary in the antileukemia activity, how this approach fares in previously treated patients who have received chemotherapy and may have a depleted immune repertoire remains to be seen. These and other questions will need to be addressed, but the findings here provide an interesting way to refocus an antileukemia immune response.
Conflict-of-interest disclosure: T.M.K. has received research funding from Bristol-Myers Squibb.