One of the major goals of hemoglobinopathy research has been to devise improved pharmacologic strategies for the induction of fetal hemoglobin (HbF) in people with sickle cell disease and β-thalassemia. In this issue of Blood, Dulmovits and colleagues report that pomalidomide, a drug approved by the US Food and Drug Administration (FDA) for treatment of multiple myeloma, induces HbF production by decreasing levels of several key transcriptional repressors of fetal globin gene expression.1 In addition, they show that pomalidomide induces HbF in differentiating erythroid cells from people with sickle cell disease and in myeloma patients.
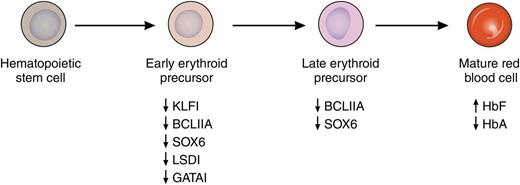
Induction of HbF by pomalidomide. Treatment with pomalidomide during in vitro differentiation of primary human erythroid cells leads to the concerted suppression of several known repressors of fetal globin gene expression. This suppression is noted at both the RNA and protein levels and is most pronounced early in differentiation. This leads to increased levels of HbF and decreased levels of adult hemoglobin (HbA) in mature erythrocytes. Professional illustration by Patrick Lane, ScEYEnce Studios.
Induction of HbF by pomalidomide. Treatment with pomalidomide during in vitro differentiation of primary human erythroid cells leads to the concerted suppression of several known repressors of fetal globin gene expression. This suppression is noted at both the RNA and protein levels and is most pronounced early in differentiation. This leads to increased levels of HbF and decreased levels of adult hemoglobin (HbA) in mature erythrocytes. Professional illustration by Patrick Lane, ScEYEnce Studios.
The observations that underlie the work of Dulmovits et al1 (for reviews, see Sankaran2 and Mabaera et al3 ) date to the early 1970s when Perrine and colleagues reported that people with homozygous sickle cell disease and a “genetically determined ability to produce large amounts of fetal haemoglobin” had remarkably benign clinical courses.4 Similar outcomes were seen for people with homozygous β-thalassemia who expressed high levels of HbF. These effects were subsequently shown to be due to the ability of HbF to inhibit sickle hemoglobin polymerization, and, in β-thalassemia, to decrease the degree of globin chain imbalance. Following the suggestion that fetal globin gene expression and HbF production might be inducible in differentiating adult erythroid cells, ground-breaking studies, first using 5-azacytidine and later hydroxyurea (HU), demonstrated that this could be accomplished and provide clinical benefits to patients. Although these studies led to HU becoming the first, and so far only, FDA-approved HbF-inducing agent, HU is not an ideal drug because blood counts of patients must be closely monitored, it is effective only in roughly half of adult sickle cell patients and in fewer β-thalassemia patients, and, for a variety of reasons, it has been underutilized in both the United States and Africa. This has prompted continued research to identify HbF-inducing agents that demonstrate long-term safety, high response rates, and ease of use such that they can be applied to most patients, including those who lack access to modern medical facilities. Although many active agents have been described, so far none have met these key criteria or achieved FDA approval. After 2 previous studies showing that pomalidomide is capable of inducing HbF in cell culture and in a murine model of sickle cell disease,5,6 Dulmovits and colleagues now provide important new data on pomalidomide’s mechanism of action and activity in humans.
A major impediment to the development of more successful HbF-inducing agents has been an incomplete understanding of how the fetal globin genes are silenced in adult erythroid cells. However, in the last decade, many of the transcription factors that mediate this silencing have been identified and include BCL11A, KLF1, SOX6, GATA1, and LSD1. As recently reviewed by Bauer and Orkin,7 KLF1 is a direct activator of BCL11A gene expression, whereas SOX6, GATA1, and LSD1 are members of repressive complexes that are targeted to the human β-globin gene locus by BCL11A. As reported in this Blood article, pomalidomide has the remarkable ability to suppress messenger RNA and protein levels of each of these factors during erythroid differentiation, leading to increased HbF and decreased adult hemoglobin levels in cells derived from normal donors and people with sickle cell disease (see figure). In their experiments, HbF was increased from 5% to more than 30% at pomalidomide doses that did not affect the terminal differentiation of erythroid cells. In cells from people with sickle cell disease, a similar increase in HbF was observed, as well as an ∼40% decrease in sickle hemoglobin. These results compare with only a 2-fold increase in HbF with HU in the same system. An important remaining question from these experiments is how pomalidomide coordinately downregulates multiple γ-globin gene repressors. Finally, the authors move beyond in vitro experiments to show that standard doses of pomalidomide (2-4 mg/d) increase γ-globin protein levels in the peripheral blood of myeloma patients, and that the levels appear to increase with longer treatment.
Despite the promise of these results, it must be noted that known risks of pomalidomide include embryo-fetal toxicity, thrombosis, and pancytopenia.8 In addition, the related drug lenalidomide has been associated with increased risk of secondary cancers in myeloma patients on maintenance therapy after autologous stem cell transplant.9 These safety issues will need to be carefully considered before moving toward clinical trials with pomalidomide. It is hoped that through additional research and better understanding of the underlying mechanism of action of this drug, the development of HbF-inducing agents with the benefits, but not the potential drawbacks, of pomalidomide can be achieved.
Conflict-of-interest disclosure: The author declares no competing financial interests.