Key Points
A novel CD3×CD123 DART agent induces T-cell-target-specific association, activation, and proliferation.
The CD3×CD123 DART induces a dose-dependent killing of AML cell lines and primary AML blasts in vitro and in vivo.
Abstract
T-cell–directed killing of tumor cells using bispecific antibodies is a promising approach for the treatment of hematologic malignancies. Here we describe our preclinical work with a dual-affinity retargeting (DART) molecule generated from antibodies to CD3 and CD123, designed to redirect T cells against acute myeloid leukemia blasts. The CD3×CD123 DART (also referred to as MGD006/S80880) consists of 2 independent polypeptides, each composed of the VH of 1 antibody in tandem with the VL of the other antibody. The target antigen CD123 (interleukin 3RA) is highly and differentially expressed in acute myeloid leukemia (AML) blasts compared with normal hematopoietic stem and progenitor cells. In this study we demonstrate that the CD3×CD123 DART binds to both human CD3 and CD123 to mediate target-effector cell association, T-cell activation, proliferation, and receptor diversification. The CD3×CD123 DART also induces a dose-dependent killing of AML cell lines and primary AML blasts in vitro and in vivo. These results provide the basis for testing the CD3×CD123 DART in the treatment of patients with CD123+ AML.
Introduction
T-cell–redirected killing of tumor cells represents a promising immunologic approach for the treatment of hematologic malignancies. Bispecific antibodies (BsAbs) combine antigen recognition sites from 2 antibodies, allowing simultaneous binding to 2 different epitopes on the same or different antigens. Several BsAb formats can redirect polyclonal T cells against tumor cells through binding to the tumor antigen and the T-cell coreceptor molecule CD3 (for review, see Byrne et al1 ). This interaction induces activation and cytotoxicity of the T effector cells against targets in an major histocompatibility complex-independent manner, thus bypassing an immune escape mechanism of major histocompatibility complex downregulation by tumor cells.
Dual-affinity retargeting (DART) proteins are a class of BsAbs that consists of 2 peptides, each composed of the variable heavy chain region of 1 antigen recognition site linked to the variable light chain region of a second antigen recognition site (supplemental Figure 1, available on the Blood Web site).2 The resultant heterodimer is stabilized by a C-terminal disulfide bond between the 2 chains. CD19×T-cell receptor (TCR) and CD19×CD3 DARTs have demonstrated in vitro killing of B-cell lymphomas by human T cells or peripheral blood mononuclear cells (PBMCs).3 Compared with other bispecific antibodies, the DART platform possesses a number of potential advantages that may enhance its clinical efficacy. The interchain disulfide bridge limits the freedom of the Fv domain components to undergo domain exchange, resulting in high stability.2,3 In a direct comparison between a CD19×CD3 DART and bispecific T-cell engager molecule constructed with identical Fv sequences, the DART outperformed the bispecific T-cell engager with respect to the magnitude of induction of markers of T-cell activation and the concentration required for lysis of B cells, effects that may be a result of the more compact configuration of the DART, as reflected in the ability of the DART to cross-link T cells and B cells more efficiently.3
In contrast to B-cell malignancies, the development of BsAbs in acute myeloid leukemia (AML) has been limited by the lack of suitable tumor-associated antigens. CD123, the interleukin 3 (IL-3) receptor α-chain (IL3RA), is normally expressed on some endothelial cells, monocytes, plasmacytoid dendritic cells, basophils, and myeloid progenitors.4,5 Binding of IL-3 stimulates CD123 heterodimerization with the common β-subunit of the granulocyte-macrophage colony-stimulating factor/IL-5/IL-3 receptor complex (CDw131) to induce hematopoietic progenitor cell differentiation and proliferation by phosphorylation of Janus kinase/signal transducer and activator of transcription molecules, activation of the PI3 kinase/mitogen-activated protein kinase pathway, and upregulation of antiapoptotic proteins.6,7 CD123 is differentially and significantly overexpressed in a large proportion (40%-93%) of patients with AML and has been identified as a marker of quiescent leukemic stem cells with very low or negligible expression in normal CD34+ progenitors.8,9
In this article, a CD3×CD123 DART (also referred to as MacroGenics compound MGD006 or Les Laboratoires Servier compound S80880) as a potential therapy for AML is described. This novel therapeutic agent can induce T-cell-target-specific association, T-cell activation, T-cell expansion, and T-cell-mediated CD123+ target killing in vitro and in vivo, using both human and mouse cell lines that overexpress CD123, as well as primary human AML samples.
Methods
DART design
MGD006 is a novel 58.9-kDa CD3×CD123 DART protein developed by MacroGenics, Inc. (Rockville, MD) and produced in Chinese hamster ovary cells.10 The CD3×CD123 DART molecule was constructed using humanized mouse anti-human CD3 and anti-human CD123 Fv sequences (supplemental Figure 1).10 Control DARTs were constructed in a similar manner, with the variable domain sequences of the anti-fluorescein mAb 4-4-20 replacing 1 or the other specificities.3
Flow cytometry
Full details of immunophenotyping characterization of human AML blasts are given in the supplemental Methods.
Cell lines
K562, A20, and Jurkat cell lines were obtained from the American Tissue Culture Collection (Manassas, VA). ΔU3 click-beetle red luciferase-green fluorescent protein (CBR-GFP) retrovirus was transduced into K562 and A20 cells.11 Transduced GFP-positive cells were sorted and cloned to establish the K562GFP and A20GFP cell lines. To generate the K562GFP-CD123 cell line, CD123-IRES-GFP murine stem cell virus (MSCV) was transduced into the K562GFP clone.11 Seventy-two hours after transduction, the cells were sorted by fluorescence-activated cell sorting for CD123+/GFP+ cells.
Analysis of T-cell/target cell association and T-cell proliferation
Jurkat cells (106 cells/mL) were labeled with 1 μM Violet Proliferation Dye 450 (VPD450; BD Biosciences, San Jose, CA) per manufacturer’s recommendations. Labeled Jurkat cells were then incubated 1:1 with K562GFPor K562GFP-CD123 cells ± DARTs at 25°C for 30 minutes. K562 cells overexpressing CD123 were γ-irradiated (100 Gy) and incubated with labeled T cells at a 1:1 ratio for 5 days.
In vitro cytotoxicity assays with cell lines
Human T cells were incubated with A20 or K562 cell lines, CD34+ progenitors, or CD14+CD123+ monocytes (4 × 104 total cells/well) at an effector:target [E:T] ratio of 10:1 or 1:1, along with the indicated DARTs (0.1-10 ng/mL) in Xcyte medium, supplemented with rhIL-2 (10 IU/mL) or FMS-like tyrosine kinase 3 ligand (50 ng/mL). Absolute cell counts of viable target cells were quantified by flow cytometry, using 7-aminoactinomycin D and SPHERO AccuCount fluorospheres (Spherotech Inc., Lake Forest, IL), as previously described.12 Chromium-51 release assays were performed as described previously.13
Purification, in vitro culture, and colony-forming cell assay of primary AML cells, cord blood CD34+ cells, and CD123+ monocytes
Full details are given in the supplemental Methods.
Next-generation sequencing of the TCRα and TCRβ genes
Banked PBMCs from patients with AML were grown for 5 days in the presence of CD3×CD123 DART molecules. RNA from flow cytometry-purified CD3+ T cells obtained at baseline and after DART treatment was extracted using a Qiagen RNeasy Micro Kit according to the manufacturer’s instructions. TCRα and TCRβ libraries were constructed, sequenced with the Roche 454 sequencing platform, and analyzed by iRepertoire (Huntsville, AL), as previously described.14 iRepertoire provided raw data on V, D, and J segment usage and CDR3 length.
Primary human AML and K562 xenograft models
Animal protocols were in compliance with the regulations of the Washington University School of Medicine Animal Studies Committee. Eight-week-old NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice (The Jackson Laboratory, Bar Harbor, ME) were sublethally irradiated (300 cGy) on day 0 and injected with K562GFP-CD123 cells (1.5 × 106) or primary human AML cells (5 × 106) into the tail vein on day 1 or day 5, respectively. Purified human T cells (3 × 106) were injected into the mice receiving K562GFP-CD123 cells on day 4, and DARTs (0.5 mg/kg/day) were injected on days 4 to 8. To track K562GFP-CD123 tumor growth in vivo, mice were injected intraperitoneally with 50 μg/g d-luciferin (Biosynth, Itasca, IL) and imaged as previously described.15,16 Mice injected with primary human AML samples were treated with the CD3×CD123 DART (0.5 mg/kg/day) on days 5 to 8. Peripheral blood, spleen, and bone marrow specimens were obtained 6 weeks postinjection and analyzed by flow cytometry for AML blasts and human T cells.
Statistical methods
Data were analyzed by GraphPad software Prism version 5 or SAS 9.3 (SAS Institutes, Cary, NC). One-way ANOVA analysis was applied when comparing multiple groups, whereas unpaired Student’s t-test was used for comparison of independent groups.
Results
CD3×CD123 DART induces effector-target cell association
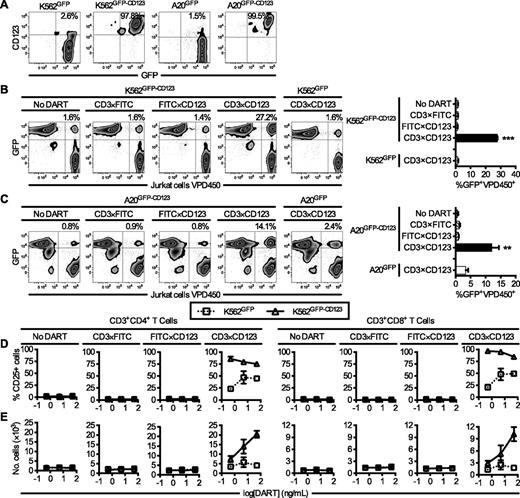
Human K562 erythroleukemia and murine A20 B cell lymphoma cell lines that express human CD123 were generated via transduction with a CD123-IRES-GFP MSCV (Figure 1A). To test the ability of the CD3×CD123 DART to redirect human T cells to target cells expressing CD123, K562GFP-CD123, and A20GFP-CD123 cells were incubated with various DARTs and VPD450-labeled Jurkat cells expressing human CD3. After exposure to the CD3×CD123 DART for 30 minutes (Figure 1B-C), the association of K562GFP-CD123 or A20GFP-CD123 cells with Jurkat cells increased by 17-fold (P < .0001) and 21-fold (P = .001), respectively, compared with untreated cells. In contrast, the CD3×CD123 DART did not induce association of Jurkat cells with cells lacking CD123 expression (K562GFP, A20GFP). Similarly, control DARTs, in which the VH and VL were replaced with variable regions of an irrelevant antigen (CD3×fluorescein isothiocyanate [FITC] and FITC×CD123 DARTs) did not induce Jurkat cell–target cell association, thus confirming the binding specificity of the CD3×CD123 DART.
The CD3×CD123 DART binds the CD3/TCR complex and human CD123 and mediates cell association and T-cell activation and expansion in vitro. (A) Expression of GFP and CD123 in K562 and A20 cell lines transduced with GFP alone or GFP-CD123. (B) K562GFP-CD123 and (C) A20GFP-CD123 cells were incubated at a 1:1 ratio with VPD450-labeled Jurkat cells in the presence of the indicated DARTs (10 ng/mL). K562GFP and A20GFP cell lines were used as controls. Association is measured by flow cytometry as the percentage of GFP+VPD450+ events divided by the total number of GFP+ and VPD450+ cells. Each bar in the summary graphs represents the average of 3 separate experiments, where samples were analyzed in duplicate in each experiment. (D-E) Human T cells (4 × 104 cells/well) were cultured with IL-2 (10 IU/mL) and irradiated K562GFP or K562GFP-CD123 cells (100 Gy) at a 1:1 ratio in the presence or absence of the indicated DARTs for 5 days. (D) Assay of T-cell activation after exposure to DARTs. An increase in CD25 expression by flow cytometry demonstrates activation of T cells in the presence of CD3×CD123 DART compared with control DARTs. (E) Assay of T-cell proliferation after exposure to DARTs. An increase in the number of CD3+ cells demonstrates proliferation of T cells in the presence of K562GFP-CD123 cells and CD3×CD123 DART compared with control DARTs and K562GFP cells. One representative example is shown out of two experiments with different donors. The data are shown as means ± standard deviations, where each point was measured in triplicate. *P < .05, **P < .01, ***P < .001.
The CD3×CD123 DART binds the CD3/TCR complex and human CD123 and mediates cell association and T-cell activation and expansion in vitro. (A) Expression of GFP and CD123 in K562 and A20 cell lines transduced with GFP alone or GFP-CD123. (B) K562GFP-CD123 and (C) A20GFP-CD123 cells were incubated at a 1:1 ratio with VPD450-labeled Jurkat cells in the presence of the indicated DARTs (10 ng/mL). K562GFP and A20GFP cell lines were used as controls. Association is measured by flow cytometry as the percentage of GFP+VPD450+ events divided by the total number of GFP+ and VPD450+ cells. Each bar in the summary graphs represents the average of 3 separate experiments, where samples were analyzed in duplicate in each experiment. (D-E) Human T cells (4 × 104 cells/well) were cultured with IL-2 (10 IU/mL) and irradiated K562GFP or K562GFP-CD123 cells (100 Gy) at a 1:1 ratio in the presence or absence of the indicated DARTs for 5 days. (D) Assay of T-cell activation after exposure to DARTs. An increase in CD25 expression by flow cytometry demonstrates activation of T cells in the presence of CD3×CD123 DART compared with control DARTs. (E) Assay of T-cell proliferation after exposure to DARTs. An increase in the number of CD3+ cells demonstrates proliferation of T cells in the presence of K562GFP-CD123 cells and CD3×CD123 DART compared with control DARTs and K562GFP cells. One representative example is shown out of two experiments with different donors. The data are shown as means ± standard deviations, where each point was measured in triplicate. *P < .05, **P < .01, ***P < .001.
CD3×CD123 DART promotes T-cell activation and proliferation
Next, T-cell activation and proliferation were examined after DART treatment. Incubation of either CD4+ or CD8+ T cells with K562GFP-CD123 in the presence of the CD3×CD123 DART resulted in a marked increase in CD25 expression (Figure 1D). Likewise, the CD3×CD123 DART selectively induced proliferation in T cells in a dose-dependent manner compared with phosphate-buffered saline (PBS) or control DART-treated cells (Figure 1E). Although incubation of the CD4+ or CD8+ T cells with control K562GFP cells in the presence of the CD3×CD123 DART also resulted in an increase in CD25 expression (Figure 1D), this T-cell activation did not result in cellular proliferation (Figure 1E). This T-cell activation induced by the control K562GFP cells was most likely caused by the small subset of K562GFP cells that expresses low levels of CD123 (Figure 1A).
CD3×CD123 DART stimulates antigen-specific cytotoxicity against CD123-expressing cell lines by donor T cells
To evaluate the ability of the CD3×CD123 DART to kill target cells in vitro, A20 (Figure 2A) or K562 cells (Figure 2B) expressing either GFP alone or both GFP and CD123 were incubated with donor T cells in the presence of the various DARTs for 1 or 2 days, and target cell survival was determined by flow cytometry. Treatment of CD123+ targets with the CD3×CD123 DART decreased the number of CD123-expressing cells relative to the GFP controls in a DART dose-dependent and statistically significant manner (Figure 2A-B). Furthermore, efficient CD3×CD123 DART-specific killing was observed when human T cells were incubated with KG1 cells, a cell line that naturally expresses the CD123 target (Figure 2C-D). No killing was observed with either of the FITC-containing control DARTs on CD123-expressing cells. Although not dose-dependent, both A20 and K562 cells expressing GFP and treated with the CD3×CD123 DART in the presence of T-cells exhibited a decrease in cell survival compared with the PBS control and FITC-containing DARTs (Figure 2A-B). This killing is most likely caused by the small subset of cells that express low levels of CD123 (Figure 1A).
The CD3×CD123 DART enhances T-cell-mediated elimination of CD123-expressing cell lines in vitro. (A-C) Human T cells (2.5 × 105 cells/well) were cultured with (A) A20GFP or A20GFP-CD123 cells, (B) K562GFP or K562GFP-CD123 cells, or (C) KG1 cells at a 10:1 ratio in the presence or absence of the indicated DARTs for 1 or 2 days. The absolute number of viable A20, K562, or KG1 targets were quantitated by flow cytometry, using 7-amino-actinomycin D (7-AAD), and cell survival is expressed relative to the PBS control. Each data point represents the average ± SD of 3 separate experiments, using different T-cell donors, where samples were analyzed in duplicate in each experiment. (D) Expression of CD123 on KG1 cells. (E-F) Human T cells were cultured with [51Cr]-labeled (E) A20GFP or A20GFP-CD123 cells or (F) K562GFP or K562GFP-CD123 at various E:T ratios in the presence of the indicated DARTs for 4 hours. *P < .05, **P < .01.
The CD3×CD123 DART enhances T-cell-mediated elimination of CD123-expressing cell lines in vitro. (A-C) Human T cells (2.5 × 105 cells/well) were cultured with (A) A20GFP or A20GFP-CD123 cells, (B) K562GFP or K562GFP-CD123 cells, or (C) KG1 cells at a 10:1 ratio in the presence or absence of the indicated DARTs for 1 or 2 days. The absolute number of viable A20, K562, or KG1 targets were quantitated by flow cytometry, using 7-amino-actinomycin D (7-AAD), and cell survival is expressed relative to the PBS control. Each data point represents the average ± SD of 3 separate experiments, using different T-cell donors, where samples were analyzed in duplicate in each experiment. (D) Expression of CD123 on KG1 cells. (E-F) Human T cells were cultured with [51Cr]-labeled (E) A20GFP or A20GFP-CD123 cells or (F) K562GFP or K562GFP-CD123 at various E:T ratios in the presence of the indicated DARTs for 4 hours. *P < .05, **P < .01.
The ability of the CD3×CD123 DART to enhance T-cell-mediated killing of CD123+ tumor cells was further assessed in a chromium release assay. Resting PBMCs isolated from healthy donors were incubated with GFP- or GFP-CD123-expressing A20 (Figure 2E) and K562 (Figure 2F) cells for 4 hours. There was significant, specific killing of the GFP-CD123-expressing targets, but not the GFP-only controls, after exposure to the CD3×CD123 DART compared with untreated cells. In contrast, there was no difference in the specific killing of targets in the presence of either of the irrelevant DARTs (data not shown). The cytotoxic effect of CD3×CD123 DART positively correlated with the effector:target ratio and DART concentration (Figure 2E-F).
CD3×CD123 DART-mediated T-cell activation and killing of primary AML blasts in vitro
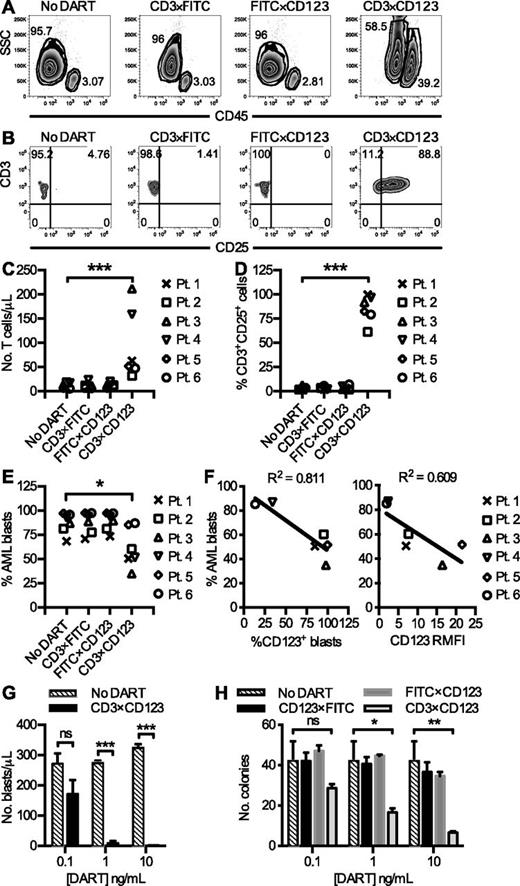
The effect of the CD3×CD123 DART on phenotypically characterized primary frozen/thawed AML samples (supplemental Table 1) was then investigated in vitro. Using only the endogenous T cells present in the frozen tumor sample as the effector cells, and not supplementing the cultures with exogenous cytokines, long-term culture assays of the primary AML samples were performed in the presence and absence of the various DARTs. Using this approach, 60% of tested patient samples (6/10) survived for 6 days in culture, with recoveries ranging from 25% to 50% in the untreated controls (data not shown). After 6 days, an increase in the relative percentage of cells in the lymphocyte gate (CD45hi, side scatterlow) was observed in response to the CD3×CD123, but not control, DARTs (Figure 3A). Flow cytometry analysis on day 6 confirmed that this response reflects T-cell expansion and activation, with an increase in CD25 expression on exposure to CD3×CD123 DART (21.4- to 152.8-fold) compared with control DARTs (0.2- to 6.5-fold), (Figures 3B-D).
The CD3×CD123 DART induces T-cell activation, expansion, and redirected killing of blasts in primary AML samples. PBMCs (2 × 105 cells/well in 96-well plate) from primary AML samples (n = 6) were incubated with DARTs at 0.1 ng/mL in the absence of exogenous cytokines for 6 days. (A) Representative flow cytometry analyses reveal an increase in the relative percentage of CD45hi lymphocytes compared with CD45dim blasts in response to CD3×CD123 DART. (B) Representative flow cytometry of CD25 expression in T cells after exposure to DARTs. (C) T-cell number and (D) CD25 expression in primary patient samples. (E) AML blast percentage after DART exposure in primary patient samples. (F) Correlation between the relative percentage of surviving blasts after exposure to CD3×CD123 DART for 6 days and baseline expression of CD123 on AML blasts. The percentage of AML cells expressing CD123 and the relative mean fluorescence intensity (RMFI) of CD123 is shown. (G) Dose-response relationship in total number of surviving blasts after exposure to CD3×CD123 DART. PBMCs (2 × 105 cells/well in 96-well plate) from AML patient 5 were incubated with PBS (No DART) or DARTs in the absence of exogenous cytokines for 6 days. Error bars represent the SD of duplicate or triplicate cultures in a single experiment. (H) Mononuclear cells from primary AML patient 1 were treated in duplicate for 6 days with DARTs 0.1 to 10 ng/mL in complete medium followed by incubation of viable cells in methylcellulose-based medium. Colonies were counted after 7 to 14 culture days. Data are representative of 2 patient samples. *P < .05, **P < .01, ***P < .001.
The CD3×CD123 DART induces T-cell activation, expansion, and redirected killing of blasts in primary AML samples. PBMCs (2 × 105 cells/well in 96-well plate) from primary AML samples (n = 6) were incubated with DARTs at 0.1 ng/mL in the absence of exogenous cytokines for 6 days. (A) Representative flow cytometry analyses reveal an increase in the relative percentage of CD45hi lymphocytes compared with CD45dim blasts in response to CD3×CD123 DART. (B) Representative flow cytometry of CD25 expression in T cells after exposure to DARTs. (C) T-cell number and (D) CD25 expression in primary patient samples. (E) AML blast percentage after DART exposure in primary patient samples. (F) Correlation between the relative percentage of surviving blasts after exposure to CD3×CD123 DART for 6 days and baseline expression of CD123 on AML blasts. The percentage of AML cells expressing CD123 and the relative mean fluorescence intensity (RMFI) of CD123 is shown. (G) Dose-response relationship in total number of surviving blasts after exposure to CD3×CD123 DART. PBMCs (2 × 105 cells/well in 96-well plate) from AML patient 5 were incubated with PBS (No DART) or DARTs in the absence of exogenous cytokines for 6 days. Error bars represent the SD of duplicate or triplicate cultures in a single experiment. (H) Mononuclear cells from primary AML patient 1 were treated in duplicate for 6 days with DARTs 0.1 to 10 ng/mL in complete medium followed by incubation of viable cells in methylcellulose-based medium. Colonies were counted after 7 to 14 culture days. Data are representative of 2 patient samples. *P < .05, **P < .01, ***P < .001.
Despite very low E:T (T cell:blast) ratios ranging from 1:10 to 1:110 (supplemental Table 1), there was a reduction in the relative percentage of AML blasts by 25.5% (P = .02) compared with the no DART control after 6 days of culture in the absence of exogenous cytokines (Figure 3E). This reduction in blast percentage and accompanying increase in the side scatter profile (Figure 3A) is suggestive of AML blast cell death after exposure to the CD3×CD123 DART and correlated with AML blast CD123 expression (Figure 3F). There was no significant correlation between the baseline number of CD3+ T cells per AML blast sample (ie, E:T ratio), the relative percentages of CD4+ and CD8+ T cells, nor AML blast PD-L1 (CD274) expression and the efficiency of DART-mediated killing in these primary human AML samples (supplemental Figure 2).
The efficiency of killing of primary human AML blasts was dependent on the concentration of CD3×CD123 DART, increasing from 27 ± 18.4% at 0.1 ng/mL to 99.6 ± 0.05% at 10 ng/mL of DART (P = .0063) (Figure 3G). Furthermore, pretreating primary AML cells with the CD3×CD123 DART resulted in significant inhibition of colony-forming capacity in methylcellulose medium. There was a dose-dependent reduction of leukemic CFUs after treatment with the CD3×CD123 DART from 32.1 ± 0.11% at 0.1 ng/mL to 85 ± 0.05% at 10 ng/mL (Figure 3H; P = .01), but not with the control DARTs (P = .91).
CD3×CD123 DART-mediated killing of primary AML blasts in the presence of stroma
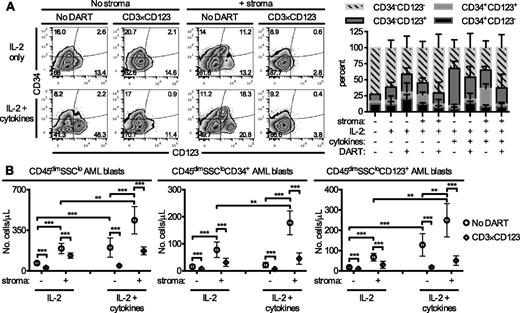
Because keeping primary AML cells alive for 6 days in the absence of cytokine and/or stroma support remains a challenge,17 the effect of the CD3×CD123 DART on banked primary AML samples cultured in the presence of stroma and IL-2 alone or IL-2 and a mixture containing macrophage colony-stimulating factor, IL-3, IL-6, thrombopoietin, and FMS-like tyrosine kinase 3 ligand (FLT3L) was evaluated. The presence of stroma increased the absolute numbers of AML cells, particularly CD34+ blasts, after coculture with either IL-2 alone or the cytokine mixture (Figure 4). Addition of the cytokine mixture to IL-2 in the presence of stroma further increased the relative percentage of CD34+CD123+ AML blasts within the cocultures. Treatment with the CD3×CD123 DART significantly decreased the absolute number of total CD45dimSSClo blasts, as well as the CD34+ and CD123+ AML subsets (Figure 4B). Similar results in the CD34+ and CD123+ AML blast subsets were obtained with samples from patient 6 (supplemental Figure 3).
The CD3×CD123 DART induces redirected killing of human AML blasts in the presence and absence of stroma. Cells from AML patient 9 were grown in the presence or absence of NSG stroma in media supplemented with IL-2 or a mixture of IL-2 and murine macrophage-colony-stimulating factor (100 ng/mL), murine IL-3, human IL-6, murine thrombopoietin, and human FLT3L. Twelve hours after initiation of culture, PBS (No DART) or 1 ng/mL CD3×CD123 DART were added to the wells. Six days later, cells were harvested and analyzed by flow cytometry for CD45dimSSClo AML blasts expressing CD34 and CD123. (A) Representative flow cytometry of CD34 and CD123 expression on CD45dimSSClo AML blasts. Bar graph represents the percentage of cells of the indicated phenotype out of all blasts in the wells. (B) The absolute numbers of total CD45dimSSClo AML blasts and CD45dimSSClo AML cells expressing CD34 and CD123. Each data point represents the average of 4 separate experiments, where samples were analyzed in duplicate or triplicate in each experiment. The average number of cells under different culture conditions were summarized using means and standard deviations and compared by two-way ANOVA for repeated measurement data. As a result of relatively large variability in data, a logarithm transformation was performed to better satisfy the assumption of normal distribution. All the tests were two-sided, and a P-value of .05 or less was taken to indicate statistical significance. *P < .05, **P < .01, ***P < .001.
The CD3×CD123 DART induces redirected killing of human AML blasts in the presence and absence of stroma. Cells from AML patient 9 were grown in the presence or absence of NSG stroma in media supplemented with IL-2 or a mixture of IL-2 and murine macrophage-colony-stimulating factor (100 ng/mL), murine IL-3, human IL-6, murine thrombopoietin, and human FLT3L. Twelve hours after initiation of culture, PBS (No DART) or 1 ng/mL CD3×CD123 DART were added to the wells. Six days later, cells were harvested and analyzed by flow cytometry for CD45dimSSClo AML blasts expressing CD34 and CD123. (A) Representative flow cytometry of CD34 and CD123 expression on CD45dimSSClo AML blasts. Bar graph represents the percentage of cells of the indicated phenotype out of all blasts in the wells. (B) The absolute numbers of total CD45dimSSClo AML blasts and CD45dimSSClo AML cells expressing CD34 and CD123. Each data point represents the average of 4 separate experiments, where samples were analyzed in duplicate or triplicate in each experiment. The average number of cells under different culture conditions were summarized using means and standard deviations and compared by two-way ANOVA for repeated measurement data. As a result of relatively large variability in data, a logarithm transformation was performed to better satisfy the assumption of normal distribution. All the tests were two-sided, and a P-value of .05 or less was taken to indicate statistical significance. *P < .05, **P < .01, ***P < .001.
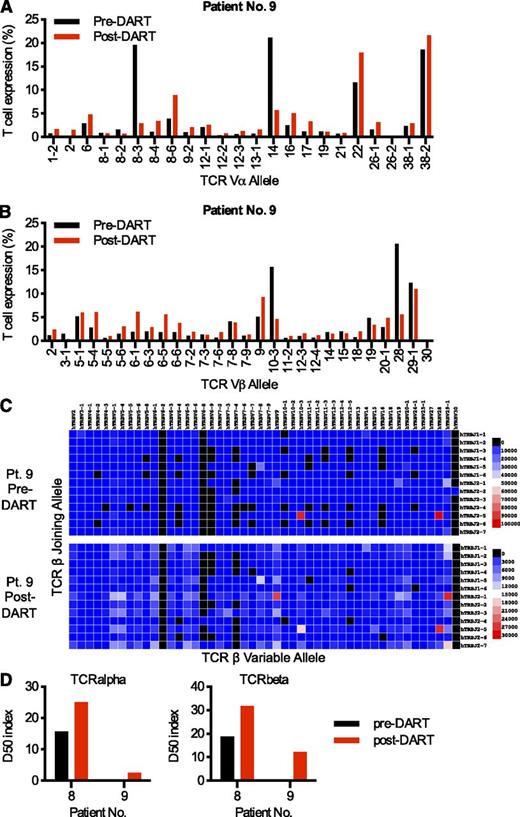
Increased TCR diversity after CD3×CD123 DART treatment
To investigate the diversity and quality of the TCR during DART treatment, next-generation sequencing was applied to samples from patients 8 and 9 with AML. The total and unique sequences of rearranged TCRα and TCRβ products for the samples are reported in supplemental Table 2. TCRα and TCRβ chain diversification were observed after 6 days of CD3×CD123 DART treatment ex vivo (Figure 5A-B; supplemental Figure 4A-B). A representative 2-dimensional heat map of the relative frequency of the TCRβ germline V-gene allele plotted relative to the germline J-gene allele for human patient 9 with AML demonstrates the dramatic increase in TCRβ diversification after DART treatment (Figure 5C). This diversification is quantified through the use of an index, D50, which is the percentage of dominant T-cell clones that account for the cumulative 50% of the total CDR3s in the sample. A more diverse library has a value closer to 50, whereas low D50 values are associated with decreased diversity. The TCRα and TCRβ D50 index increased for both patients with AML after CD3×CD123 DART treatment (Figure 5D).
The CD3×CD123 DART induces TCR diversification in primary AML samples. Banked PBMCs from AML patients 8 and 9 were grown for 5 days in the presence of CD3×CD123 DART molecules. Purified T cells obtained at baseline (pre-DART) and after DART treatment (post-DART) were evaluated by next-generation sequencing of the TCR-Vα and TCR-Vβ families. Relative levels of 23 specific TCR-Vα (A) and 28 specific TCR-Vβ (B) families are shown here for AML patient 9 and in supplemental Figure 4 for patient 8. These genes were selected for presentation based on being expressed in more than 1% of the T cells in either of the patient samples. (C) TCRβ gene usage. A heat map of the relative frequency of a germline TCR variable (V)-gene allele is plotted relative to the germline joining (J)-gene allele for AML patient 9. The frequency of each V-J combination is represented by the color of the map. (D) D50 index. The D50 index is a quantitative measure of the degree of diversity of T cells within a sample. The D50 is the percentage of T-cell clones that account for the cumulative 50% of the total CDR3s counted in the sample. The more diverse a library, the closer the value will be to 50. Low diversity values are associated with decreased diversity. The data suggest that TCRβ and TCRα diversity increased after DART treatment of both patients.
The CD3×CD123 DART induces TCR diversification in primary AML samples. Banked PBMCs from AML patients 8 and 9 were grown for 5 days in the presence of CD3×CD123 DART molecules. Purified T cells obtained at baseline (pre-DART) and after DART treatment (post-DART) were evaluated by next-generation sequencing of the TCR-Vα and TCR-Vβ families. Relative levels of 23 specific TCR-Vα (A) and 28 specific TCR-Vβ (B) families are shown here for AML patient 9 and in supplemental Figure 4 for patient 8. These genes were selected for presentation based on being expressed in more than 1% of the T cells in either of the patient samples. (C) TCRβ gene usage. A heat map of the relative frequency of a germline TCR variable (V)-gene allele is plotted relative to the germline joining (J)-gene allele for AML patient 9. The frequency of each V-J combination is represented by the color of the map. (D) D50 index. The D50 index is a quantitative measure of the degree of diversity of T cells within a sample. The D50 is the percentage of T-cell clones that account for the cumulative 50% of the total CDR3s counted in the sample. The more diverse a library, the closer the value will be to 50. Low diversity values are associated with decreased diversity. The data suggest that TCRβ and TCRα diversity increased after DART treatment of both patients.
Effect of CD3×CD123 DART on survival of normal progenitors and monocytes
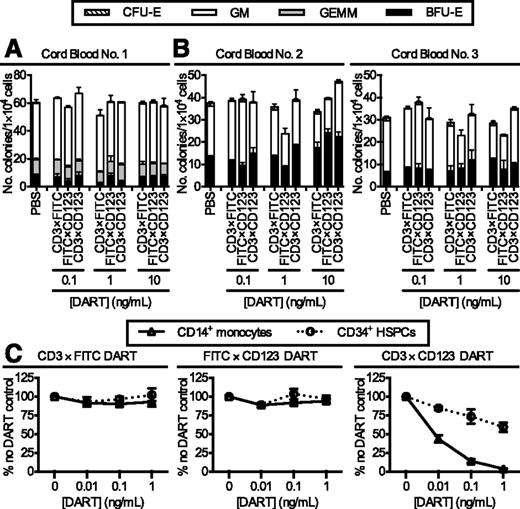
To assess the potential effect of the DARTs on normal hematopoietic progenitors and other CD123-expressing hematopoietic cells, the CD3×CD123 and control DARTs were tested in both methylcellulose colony-forming assays and short-term liquid culture. The CD3×CD123 DART had no effect on colony formation after in vitro culture of cord blood CD34+ progenitors for 4 hours (Figure 6A) or 18 hours (Figure 6B). In liquid culture, the CD3×CD123 DART exhibited minimal effect after 18- to 24-hour incubation with CD34+ progenitors, but a drastic decrease in CD14+CD123+ monocytes compared with treatment with a FITC×CD123 control DART (Figure 6C).
Effect of CD3×CD123 DART on normal CD34+ progenitor cells. Cord blood cells from 3 healthy donors were incubated with DARTs for either (A) 4 hours or (B) 18 hours and plated in methylcellulose-based medium. Colonies were scored on day 7. Error bars represent the SD of duplicate plates. (C) Purified CD34+ progenitors and CD14+CD123+ monocytes from a healthy donor mobilized peripheral blood product (n = 2) were incubated with DARTs and autologous T cells for 18 hours. Cell survival was determined by flow cytometry.
Effect of CD3×CD123 DART on normal CD34+ progenitor cells. Cord blood cells from 3 healthy donors were incubated with DARTs for either (A) 4 hours or (B) 18 hours and plated in methylcellulose-based medium. Colonies were scored on day 7. Error bars represent the SD of duplicate plates. (C) Purified CD34+ progenitors and CD14+CD123+ monocytes from a healthy donor mobilized peripheral blood product (n = 2) were incubated with DARTs and autologous T cells for 18 hours. Cell survival was determined by flow cytometry.
Antileukemic effect of CD3×CD123 DART in vivo
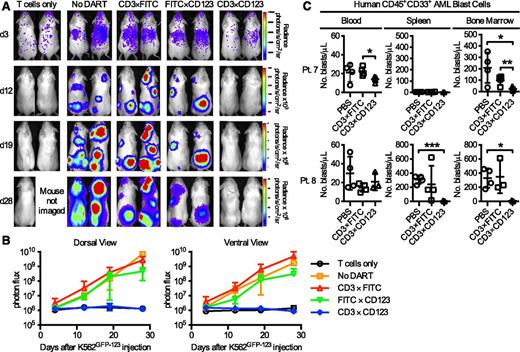
The antileukemic effect of the CD3×CD123 DART was tested in vivo, using both K562 cell lines and primary AML blasts. In the first model, 3 × 106 isolated resting human T cells were transplanted intravenously on day 4 into sublethally irradiated NSG mice bearing K562GFP−CD123 tumor cells that overexpress click-beetle red luciferase (CBRLuc). Tumor burden was followed via serial bioluminescent imaging (Figure 7A). There was an average 1415-fold increase in photon flux from accumulating leukemic cells in untreated mice when compared with those treated with CD3×CD123 DART (Figure 7B; P < .0001). No effect on tumor growth was seen in mice receiving the control DARTs. CBRluc signal emission in treated mice was comparable to that of the mice lacking tumor cells, reflecting nearly complete suppression of tumor growth in response to CD3×CD123 DART for the duration of follow-up (Figure 7A-B).
CD3×CD123 DART suppresses CD123+ leukemia xenograft in NSG mice. (A-B) Irradiated NSG mice (n = 5/group) injected with K562GFP-CD123 cells and treated with DARTs. Bioluminescence imaging on days 3, 12, 19, and 28 showed significant inhibition (P < .0001) of tumor growth in CD3×CD123 DART-treated cells compared with CD3×FITC, FITC×CD123 control DARTs, or no DART (PBS). (C) NSG mice (n = 3-4/group) were sublethally irradiated (300 cGy) on day 0 and injected with primary human AML cells (5 × 106 cells/mouse) from patient number 7 or 8 on day 5. Mice were treated with PBS, CD3×FITC control DART, or CD3×CD123 DART (0.5 mg/kg/day) on days 5 to 8. Peripheral blood, spleen, and bone marrow specimens were obtained 6 weeks after injection and analyzed by flow cytometry for CD45+CD33+ AML blasts. Data represent the mean and ±SD. *P < .05, **P < .01, ***P < .001.
CD3×CD123 DART suppresses CD123+ leukemia xenograft in NSG mice. (A-B) Irradiated NSG mice (n = 5/group) injected with K562GFP-CD123 cells and treated with DARTs. Bioluminescence imaging on days 3, 12, 19, and 28 showed significant inhibition (P < .0001) of tumor growth in CD3×CD123 DART-treated cells compared with CD3×FITC, FITC×CD123 control DARTs, or no DART (PBS). (C) NSG mice (n = 3-4/group) were sublethally irradiated (300 cGy) on day 0 and injected with primary human AML cells (5 × 106 cells/mouse) from patient number 7 or 8 on day 5. Mice were treated with PBS, CD3×FITC control DART, or CD3×CD123 DART (0.5 mg/kg/day) on days 5 to 8. Peripheral blood, spleen, and bone marrow specimens were obtained 6 weeks after injection and analyzed by flow cytometry for CD45+CD33+ AML blasts. Data represent the mean and ±SD. *P < .05, **P < .01, ***P < .001.
In a second model, NSG mice were sublethally irradiated (300 cGy, n = 3-4 mice/group) on day 0, and then primary AML samples from patients 7 and 8 (5 × 106 cells/mouse) were adoptively transferred into the mice on day 5, followed by DART infusion (0.5 mg/kg) from days 5 to 8. In this experiment, effector cells consisted of endogenous T cells present in the tumor samples with no additional T cells added. AML engraftment in the PBS and/or CD3×FITC DART control cohorts was heterogeneous at 6 weeks after transplantation, with blast cells from patient 7 demonstrating no splenic engraftment (Figure 7C). Despite very low initial E:T (T cell:blast) ratios of 1:6 to 1:42 (supplemental Table 1), there was a near-complete eradication of blasts in the bone marrow (patients 7 and 8) and spleen (patient 8) of NSG mice treated with the CD3×CD123 DART (Figure 7C). In general, very small numbers of circulating AML blasts were observed in the NSG mice. Similarly, very few human CD3+CD4+ and CD3+CD8+ T cells were detected in the NSG recipients injected with the 2 different primary AML samples (supplemental Figure 5), with no evidence of significant cytokine (IL-2, IL-4, IL-6, IL-10, interferon γ, or tumor necrosis factor α) release into the plasma at 4 hours after the first and last DART treatment (supplemental Figure 6).
Discussion
In this article, the preclinical activity of a CD3×CD123 DART was described for patients with AML. It was shown that the CD3×CD123 DART recognizes CD123+ leukemia cells and induces T-cell activation and expansion with subsequent killing of CD123-expressing tumor cells both in vitro and in vivo. Because morbidity and mortality associated with allogeneic transplant limits its clinical use, the development of nontransplant-based immunotherapeutic approaches is attractive in AML.
CD123 was initially identified as a potential target for AML on the basis of its description as a putative marker for leukemic stem cells based on its surface expression in AML blasts that engraft in severe combined immunodeficiency mice. A diphtheria toxin-IL3 conjugate that binds to CD123 has been tested in clinical trials in AML with modest activity, but its use is hampered by the immunogenicity of diphtheria toxin and symptoms of capillary leak.18 Monoclonal antibodies to CD123 are also being developed in AML. First-generation antibodies that bind and block IL-3 signaling, but do not mediate antibody-dependent cellular cytotoxicity (ADCC), were found to be safe and specific, but lacked clinical activity.19 Clinical studies using second-generation CD123 antibodies that have been optimized to enhance ADCC are currently ongoing. Single-chain Fv antibody fragments to CD123 have been generated and demonstrated to induce ADCC in CD123-expressing cell lines.20 In addition, Kuo et al21 demonstrated in in vitro studies the antileukemic activity of a CD3×CD123 bispecific scFv immunofusion construct, but nonspecific activation of T cells and interferon γ secretion were noted at low concentrations. Furthermore, the murine anti-CD3 sequence used raises a concern of an increased immunogenicity in humans.21 Finally, both bispecific (CD123×CD16) and trispecific (CD123×CD16×CD33 and CD123×CD16×CD123) single-chain Fv reagents capable of redirecting NK cells (via CD16) to kill tumor targets have also been developed and tested.20,22 Although NK cell-mediated ADCC has been demonstrated in vitro against AML cells, these reagents still require humanization to reduce immunogenicity, and their in vivo activity has yet to be reported.
CD123–chimeric antigen receptor (CAR) T cells, as well as CD123-CAR cytokine-induced killer cells (in which PBMCs are activated with interferon γ and IL-2), have been generated as potential therapeutic agents in AML. Both have demonstrated significant cytocidal activity against both CD123-expressing cell lines and primary AML cells in vitro and in vivo.23,24 Bispecific antibodies, such as the CD3×CD123 DART, possess important differences from CAR-T and cytokine-induced killer cells that may potentially make them more attractive in AML. In B-cell malignancies, persistence of CAR-T cells after infusion may maintain remission by continuing to eliminate residual malignant CD19+ B cells, but it may also induce a prolonged state of B lymphopenia by continuing to eliminate normal B cells. In AML, persistence of T cells with anti-CD123 specificity may result in cumulative suppressive effects on normal hematopoiesis, resulting in prolonged pancytopenia. The use of CAR-T cells requires retroviral transduction of cells from individual patients (or HLA-matched allogeneic stem cell donors), which presents technical and logistical challenges for widespread clinical use.
There are important potential limitations in this study, both in targeting CD123 in AML and in assessing the CD3×CD123 DART as a therapeutic agent. CD123, although highly expressed on AML blasts, is also expressed on plasmacytoid dendritic cells, monocytes, and basophils, and at low levels on normal hematopoietic stem and progenitor cells.4 Recent data with a CD123-CAR using NSG mice engrafted with human CD34+ cells resulted in the ablation of normal hematopoiesis and reduced myeloid colony formation of cord blood-derived CD34+ cells.25 However, other groups have reported little to no effect of a CD123 CAR on hematopoietic stem and progenitors either in vitro or in vivo.24,26 The CD3×CD123 DART used here cross-reacts with cynomolgus monkey CD123 and CD3, was well tolerated when administered continuously to monkeys at 1 μg/kg/day for up to 4 weeks, and was rapidly cleared after the end of infusion, with a mean residence time of 4 hours.10 The results also showed DART-dependent depletion of circulating plasmacytoid dendritic cells, but only limited and reversible effects on bone marrow progenitor cells.10 Consistent with these data, we found that the CD3×CD123 DART had little effect on normal hematopoietic stem and progenitor cell colony formation after in vitro culture.
Finally, there are important effects of the DART on T-cell number, phenotype, activation, and proliferation that are dependent on target (CD123) binding. Both bispecific T-cell engagers and DARTs redirect cytotoxic T cells against antigens in an HLA-independent fashion via formation of artificial T-cell synapses. These artificially induced synapses resemble physiological immunological synapses and induce activation of the effector T cells, followed by cytotoxic responses mediated through release of perforin and granzyme and increased expression of Fas ligand and cytokine secretion.27-29 Previous studies suggest an impaired ability of T cells in leukemic patients to form functional immunological synapses with autologous antigen-presenting cells.30 Even with extremely low effector:target ratios (<1:100) in the primary AML specimens, treatment with the CD3×CD123 DART still resulted in dramatic expansion and diversification of T cells in vitro and robust killing of CD123+ AML targets in vitro and in vivo. Our observation of low human T cell numbers in the NSG mice injected with primary human AML samples and treated with either PBS or the control CD3×FITC DART (Figures 7C; supplemental Figure 5) was expected, given the very small numbers of human T cells present in these banked AML samples (E:T ratio ranged from 1:6 to 1:42) and injected into the NSG mice. In contrast, given the clearance of AML blasts from the bone marrow and spleen of NSG mice treated with the CD3×CD123 DART, the data suggest the human T cells became activated and killed the AML blast targets in these groups of NSG mice. The lack of human T cells in these CD3×CD123 DART cohorts is mostly likely a result of T-cell contraction on elimination of the CD123+ AML blast targets. Overall, the in vitro and in vivo preclinical data generated in these studies provide a compelling rationale for testing the CD3×CD123 DART in patients with AML. The first in-human phase I study for patients with relapsed or refractory AML is currently underway.
Presented in abstract form at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 9, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri, for the use of their core facilities. The Siteman Cancer Center is supported in part by Cancer Center Support Grant P30 CA91842 from the National Cancer Institute at the National Institutes of Health.
This work was supported in part by The Foundation for Barnes-Jewish Hospital and Siteman Cancer Center and National Cancer Institute grants P01 CA101937 (principal investigator: Timothy J. Ley), P50 CA171963 (principal investigator: Daniel C. Link), R01 CA194552 (principal investigator: J.F.D.), and P50 CA94056 to the Imaging Core of the Siteman Cancer Center at Washington University. G.L.U. is supported by National Cancer Institute grant K23 CA140707.
Authorship
Contribution: M.A.-H. and M.P.R. designed and performed research; collected, analyzed, and interpreted data; performed statistical analysis; and wrote the manuscript. J.K.R. and D.K. designed and performed research; collected, analyzed, and interpreted data; and performed statistical analysis. L.G.E. and G.L.U. analyzed and interpreted data and wrote the manuscript. L.C. performed research and collected and analyzed data. F.G. performed statistical analyses. E.B., G.R.C., P.A.M., and S.J., contributed the DART reagents. W.C.E. contributed analytical tools. J.F.D. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: E.B., G.R.C., P.A.M., and S.J. are employees of MacroGenics, Inc., the company developing CD3×CD123 DART, and receive salary and stock options as compensation for their employment. The other authors declare no competing financial interests.
Correspondence: John F. DiPersio, Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8007, St. Louis, MO 63110; e-mail: jdipersi@dom.wustl.edu.
References
Author notes
M.A.-H. and M.P.R. contributed equally to this study

![Figure 2. The CD3×CD123 DART enhances T-cell-mediated elimination of CD123-expressing cell lines in vitro. (A-C) Human T cells (2.5 × 105 cells/well) were cultured with (A) A20GFP or A20GFP-CD123 cells, (B) K562GFP or K562GFP-CD123 cells, or (C) KG1 cells at a 10:1 ratio in the presence or absence of the indicated DARTs for 1 or 2 days. The absolute number of viable A20, K562, or KG1 targets were quantitated by flow cytometry, using 7-amino-actinomycin D (7-AAD), and cell survival is expressed relative to the PBS control. Each data point represents the average ± SD of 3 separate experiments, using different T-cell donors, where samples were analyzed in duplicate in each experiment. (D) Expression of CD123 on KG1 cells. (E-F) Human T cells were cultured with [51Cr]-labeled (E) A20GFP or A20GFP-CD123 cells or (F) K562GFP or K562GFP-CD123 at various E:T ratios in the presence of the indicated DARTs for 4 hours. *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/1/10.1182_blood-2014-05-575704/5/m_122f2.jpeg?Expires=1770999692&Signature=u~D7qh85ETLMJvBypyavAgd4122R2GhgwLGwcj~kzuybkkIwu8KLNMkefLd~sU84jwjWwh7wHyLFAd2Fk3q0D5B1kZq~1CT5~n9Hr99WkUDGBgKS70KVMn1pPJLAC-mTGFds7cGTola4Qo6NZt-71L~2M-tbLQ37KukWwoK8O7sBlui5M8Moom0GDjpy~O0lkC4aulSu2C03ePMBL7JGeoWOqBkZy6w~tchch-DU5k214Ia2T5cVJTAjdfttkxbjaMRZkyEkuTL5yxuZP3uZU0gMP8lEpG2fDjcxj4BQopTWuGI73xmlDp7kDg01BLIH49y2liep-cIfKSOFcSfvBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)