Key Points
We generated a novel monovalent anti-FcγRIII/albumin fusion protein that ameliorates antibody-mediated murine ITP.
Severe adverse events by anti-FcγR antibodies because of FcγR cross-linking are overcome by monovalent FcγR blockade.
Abstract
Patients with immune thrombocytopenia (ITP) commonly have antiplatelet antibodies that cause thrombocytopenia through Fcγ receptors (FcγRs). Antibodies specific for FcγRs, designed to inhibit antibody-FcγR interaction, had been shown to improve ITP in refractory human patients. However, the development of such FcγR-specific antibodies has stalled because of adverse events, a phenomenon recapitulated in mouse models. One hypothesis behind these adverse events involved the function of the Fc region of the antibody, which engages FcγRs, leading to inflammatory responses. Unfortunately, inhibition of Fc function by deglycosylation failed to prevent this inflammatory response. In this work, we hypothesize that the bivalent antigen-binding fragment regions of immunoglobulin G are sufficient to trigger adverse events and have reasoned that designing a monovalent targeting strategy could circumvent the inflammatory response. To this end, we generated a fusion protein comprising a monovalent human FcγRIIIA-specific antibody linked in tandem to human serum albumin, which retained FcγR-binding activity in vitro. To evaluate clinically relevant in vivo FcγR-blocking function and inflammatory effects, we generated a murine version targeting the murine FcγRIII linked to murine albumin in a passive murine ITP model. Monovalent blocking of FcγR function dramatically inhibited antibody-dependent murine ITP and successfully circumvented the inflammatory response as assessed by changes in body temperature, basophil activation, and basophil depletion. Consistent with our hypothesis, in vivo cross-linking of the fusion protein induced these inflammatory effects, recapitulating the adverse events of the parent antibody. Thus, monovalent blocking of FcγR function demonstrates a proof of concept to successfully treat FcγR-mediated autoimmune diseases.
Introduction
Autoimmune antiplatelet antibodies are prevalent among patients with immune thrombocytopenia (ITP).1-5 Antibody-mediated platelet destruction in the majority of ITP patients involves Fc-mediated phagocytosis by macrophages via the Fcγ receptors (FcγRs).2-4 One of the major activatory FcγRs implicated in platelet depletion is the FcγRIIIA, also a therapeutic target.6 The first FcγRIIIA-specific monoclonal antibody (mAb) 3G8 was described in 19827 and was later investigated clinically in ITP patients.7-9 Encouragingly, >50% of ITP patients refractory to other treatments responded with significantly improved platelet counts.8,10 However, its continued therapeutic application was stalled by adverse events, including vomiting, nausea, and fever.8,10,11
One major hypothesis behind the adverse events involves the (anti-FcγRIIIA antibody) Fc-mediated interaction with FcγRIIIA in addition to its antigen-binding fragment (Fab) domain–induced cross-linking. The cross-linking of FcγRIIIA triggers phosphorylation of the signaling components of this receptor, leading to proinflammatory responses.6 Such coordinated cross-linking, normally mediated by the Fc region of immunoglobulin (Ig) G–opsonized immune complexes, represents an important mode of immune activation.6 However, uncontrolled cross-linking of FcγRs, such as by an anti-FcγRIIIA antibody, can lead to an anaphylactic reaction,6,12,13 as evidenced by the 3G8 trial.8,10
One potential means of reducing unwanted adverse events involves abolishing Fc-mediated effector function. A deglycosylated version of 3G8 (called GMA161), known to have abrogated Fc function,14 had thus been developed by Fc engineering.15 In a humanized mouse model, GMA161 was able to ameliorate ITP, but unfortunately, rapidly depleted granulocytes.15 Consistent with the humanized mouse model, GMA161 improved platelet counts in refractory patients but failed to reverse adverse events exhibited by its parent 3G8.10,11 The outcome of the pilot trial of GMA161 suggested to us that the bivalent Fab domain likely triggers the adverse events. We have speculated here that a monovalent Fab fragment would represent a suitable therapeutic candidate. However, the small size of the Fab region (∼50 kDa) would be a known hindrance to its therapeutic applications, as the in vivo half-life of small proteins decreases as a function of their size.16,17 In fact, the Fab fragment of the anti-huFcγRIIIA 3G8 had been shown to be ineffective in ameliorating ITP in refractory patients, likely because of its very short half-life.11
In the present study, we pioneered a novel monovalent FcγR-targeting approach to circumvent the short half-life problems as well as the adverse events associated with the previous anti-FcγRIIIA therapeutics. We designed and generated a monovalent fusion protein composed of the single chain variable region (scFv) of the anti-FcγRIIIA antibody 3G8 and human serum albumin (HSA), which retained the ability to inhibit IgG-FcγRIIIA interaction in vitro. Furthermore, we generated a monovalent fusion construct for the anti-FcγRIII/IIB antibody 2.4G2, the murine counterpart of 3G8, and demonstrated its in vivo efficacy in ITP amelioration. Importantly, this monovalent construct displayed a notable lack of in vivo adverse events exhibited by its parent antibody, including decreased body temperature and basophil activation. Therefore, this work demonstrates the proof of concept of monovalent targeting to treat ITP and hints at its potential to treat other FcγR-mediated autoimmune diseases.
Methods
Mice
CD-1 female mice (Charles River Laboratories, Kingston, NY) were used for all in vivo experiments. All mice were housed with water and food ad libitum. All animal experiments were approved by the St. Michael’s Hospital Animal Care and Use Committee.
Antibodies and reagents
Rat IgG2b–fluorescein isothiocyanate (FITC) isotype control was purchased from Miltenyi Biotech, Canada. Unconjugated monoclonal anti-His antibody (HIS.H8) and AmpliTaq Gold 360 Master Mix were from Life Technologies, Canada. The unconjugated murine FcγRIII/IIB-specific 2.4G2 was from BioX Cell, and the Fab fragment of 2.4G2 was generated using the Fab preparation kit (Life Technologies). The anti-huFcγRIIIA 3G8 was from Biolegend. HSA was from Bayer, Canada. IVIg (Privigen) was from CSL Behring, Canada. Human serum IgG (huIgG, I4506) and bovine serum albumin were from Sigma, Canada. The staining buffer used for flow cytometry was phosphate-buffered saline (PBS) supplemented with 1% fetal bovine serum, 1 mM EDTA adjusted to pH 7.4. The plasmids encoding the heavy and light chains of 6A6 IgG2a were gifts from Prof Falk Nimmerjahn at the University of Erlangen-Nuremberg, Germany.
Cloning and construction of fusion protein constructs
Total RNA was extracted from the 2.4G2 hybridoma using RNeasy kit (Qiagen, Hungary), and reverse transcription was initiated with oligo dT using RevertAid kit from Fermentas, Hungary. Combinations of forward and reverse primers were tested to identify the best-fitting sequences judged by the intensity and correct size of the polymerase chain reaction (PCR) product. Light chain variable (VL) sequence was amplified by the VκBackNco-Vκ For4 pair, heavy chain variable (VH) sequence was obtained by VH5Cut-γCH3. PCR products were sequenced to confirm correct protein-coding framework. Restriction endonuclease sites and linker sequence were introduced during a second PCR step, followed by overlapping extension PCR joining the VL and VH fragments. The final 2.4G2 scFv construct had the arrangement VL-(G4S)3-VH (Table 1).
Primers for cloning 2.4G2 VL and VH
| Primer name . | Sequence . |
|---|---|
| VkBackNco | TCC ATG GAC ATT GAG CTC ACC CAG TCT CC |
| VkFor4 | TTT GAT TTC CAC CTT GGT CCC |
| VH5Cut | CAG GTA CAG CTA GTG GAG TCT GG |
| γCH3 | GGA TAG ACA GAT GGG GCT GTT G |
| Primer name . | Sequence . |
|---|---|
| VkBackNco | TCC ATG GAC ATT GAG CTC ACC CAG TCT CC |
| VkFor4 | TTT GAT TTC CAC CTT GGT CCC |
| VH5Cut | CAG GTA CAG CTA GTG GAG TCT GG |
| γCH3 | GGA TAG ACA GAT GGG GCT GTT G |
The 3G8 scFv sequence was kindly provided by Dr Jörg Brünke (University of Erlangen-Nuremberg, Germany). The 3G8 scFv-MSA construct consists of the huFcγRIIIA-binding domain [3G8 scFv in the arrangement of VL-(G4S)4-VH] fused to HSA (Uniprot P02768) via a hexa-glycine linker. The 2.4G2 scFv-MSA construct consists of the murine FcγRIII/IIB-binding domain [2.4G2 scFv in the arrangement of VL-(G4S)3-VH] fused to mouse serum albumin (MSA) (Uniprot P07724) via a hexa-glycine linker. Genes containing nucleotide sequences of the 3G8 scFv-HSA or the 2.4G2 scFv-MSA fusion construct were synthesized by GeneArt. The constructs were then cloned into the mammalian expression vector pHLSec encoding a hexahistidine tag using AgeI and KpnI (New England Biolabs, Canada) as described.18 The soluble domain of huFcγRIIIA (of the high-affinity valine158 variant) was cloned into pHLSec as previously described.18 The nucleotide sequences of all constructs were verified by sequencing (ACGT Corp., Canada).
Recombinant protein expression and purification
The 3G8 scFv-HSA, 2.4G2 scFv-MSA, huFcγRIIIA, and 6A6-IgG2a were all expressed by transient expression in HEK293T cells (a gift from Prof Jean-Philippe Julien, University of Toronto, Canada) in a similar fashion as previously described.18,19 Briefly, cells were grown to 90% confluence before transfection with polyethylenimine and switched to serum-free Dulbecco’s modified Eagle medium (GE Healthcare, Canada) during recombinant protein expression. Cell culture supernatant was harvested 5 days after transfection and filtered (0.22 µm) before protein purification. Nickel sepharose and protein G agarose (both from GE Healthcare) were used to purify histidine-tagged recombinant proteins and 6A6-IgG2a, respectively.
In vitro binding activity of 3G8 scFv-HSA
The binding activity of 3G8 scFv-HSA for huFcγRIIIA was assessed by enzyme-linked immunosorbent assay (ELISA). The huFcγRIIIA was coated onto high-binding microtiter plates (3590; Corning, Canada) at 5 µg/mL overnight at 4°C. High-binding plates are designed to allow maximal adsorption of antigen onto the well surface and are recommended for general ELISAs. To examine direct binding of 3G8 scFv-HSA for huFcγRIIIA, the plate was blocked using 1% Casein (Life Technologies) for 1 hour, followed by incubation of serial dilutions of 3G8 scFv-HSA or HSA (highest concentration: 870 nM) for 1.5 hours at room temperature. Bound 3G8 scFv-HSA was detected by anti-HSA-HRP (Abcam, Canada). To examine the ability of 3G8 scFv-HSA to inhibit huIgG binding to huFcγRIIIA, the plate was first blocked with 5% bovine serum albumin, and then serial dilutions of 3G8 scFv-HSA, HSA (both highest concentration: 650 nM), or 3G8 (highest concentration: 67 nM) were added to wells containing 0.8 µg/mL huIgG. HuIgG was premixed with these inhibitors before being adding to wells coated with huFcγRIIIA and allowed to bind for 1.5 hours at room temperature. Bound huIgG was detected by goat F(ab')2 anti-human IgG F(ab')2-HRP (Abcam, Canada). The 3,3′,5,5′-tetramethylbenzidine substrate (Life Technologies) was used for color development, and color development was stopped by adding 2 M H2SO4. Absorbance was measured at 450 nm on a Spectramax M5 plate reader (Molecular Devices, CA).
In vitro binding activity of 2.4G2 scFv-MSA to RAW264.7 macrophages
RAW264.7 macrophage-like culture cells (ATCC), known to express FcγRIIIA and FcγRIIB,20 were used to examine the in vitro specificity of 2.4G2 scFv-MSA. To examine the direct binding of 2.4G2 scFv-MSA to RAW264.7 cells, 5 × 105 cells were incubated with 0.11 µM 2.4G2 scFv-MSA (10 µg/mL) in the presence of the vehicle control (PBS) or an equimolar amount of 2.4G2 and HSA (as competitive inhibitors) for 1 hour on ice. The remaining bound 2.4G2 scFv-MSA was detected by anti-His-phycoerythrin (PE) (Miltenyi Biotech). To examine the ability of 2.4G2 scFv-MSA to inhibit the binding activity of its parent antibody 2.4G2, 0.11 µM (10 µg/mL) 2.4G2 scFv-MSA, 2.4G2, or HSA was added to 5 × 105 RAW264.7 cells in the presence of 0.013 µM (2 µg/mL) PE-labeled 2.4G2 (BD Biosciences, Canada) for 1 hour on ice, and residual bound 2.4G2-PE was quantified. MACS Quant flow cytometer (Miltenyi Biotech) was used for flow cytometry analysis, and all data were processed by Flowjo V10 software (Flowjo).
In vivo pharmacokinetics
To examine and compare the in vivo pharmacokinetics of 2.4G2 scFv-MSA and 2.4G2 Fab fragment, mice were injected intravenously with either 80 µg 2.4G2 scFv-MSA or ∼200 µg 2.4G2 Fab. The molar ratio of 2.4G2 Fab to 2.4G2 scFv-MSA is ∼4.5 to 1. These doses were selected to allow clear detection of residual 2.4G2 scFv-MSA and 2.4G2 Fab in serum 30 minutes after injection. Mice were bled (10 µL blood) via the saphenous vein 0.5, 2, 4, 8, 24, and 48 hours after injection. The serum from each time point was prepared by centrifugation and stored at −80°C before analysis. To examine the residual level of 2.4G2 scFv-MSA and 2.4G2 Fab after each time point, 2.5 × 105 RAW264.7 cells were stained with 1/50 diluted serum for 1 hour. Bound 2.4G2 scFv-MSA was detected by anti-His-PE, and bound 2.4G2 Fab was detected by anti-rat IgG-κ chain-PE (Biolegend). MACS Quant flow cytometer was used to analyze stained cell samples, and all data were processed by Flowjo V10 software.
ITP induction and therapeutic treatment
All treatments were administered intravenously via the lateral tail vein unless otherwise stated. To examine the in vivo therapeutic effect of 2.4G2 scFv-MSA, mice were pretreated with 10, 20, 40, or 80 µg of 2.4G2 scFv-MSA, 56 µg HSA (equimolar to 80 µg 2.4G2 scFv-MSA), or 25 mg IVIg (intraperitoneally) for 2 hours before induction of ITP by treatment of 2 µg MWReg30 or 3 µg 6A6-IgG2a. Mice were bled via the saphenous vein before treatment, then at 2, 24, and 48 hours after ITP induction, and the platelet number was enumerated by a Z2 particle counter (Beckman Coulter, Canada) as previously described.19
Body temperature measurement
Body temperature was used to assess the occurrence of an anaphylactic response induced by different treatments.12,13 Briefly, mice were injected intravenously with 0.43 nmol (65 µg) 2.4G2 or equimolar amount of 2.4G2 scFv-MSA or HSA. To cross-link 2.4G2 scFv-MSA before in vivo administration, a half-molar amount (0.22 nmol) of anti-His antibody was added to 0.43 nmol 2.4G2 scFv-MSA and incubated for 30 minutes at room temperature. Body (rectal) temperature was monitored 0.5, 1, 1.5, and 2 hours posttreatment using Thermocouple Thermometer, model TK-610B (Harvard Apparatus).
Basophil quantification and CD200R3 detection
The level of CD200R3 expression on basophils from peripheral blood was examined using flow cytometry as described.13,21 Briefly, mice were bled before treatment and 4 and 24 hours after treatment. Red blood cells (RBCs) were lysed by incubation with ammonium chloride buffer for 5 minutes at 37°C, and the peripheral blood mononuclear cells (PBMCs) were then stained with anti-CD49b-Pacific Blue (DX5), anti-FcεRIα-PerCP/Cy5.5 (MAR-1) (both from Biolegend), and anti-CD200R3-FITC (BA103) (Hycult Biotech, the Netherlands). Basophils were gated as FcεRIα positive, CD49b dim cells and confirmed with CD200R3 expression. The control blood basophil concentrations calculated in this experiment were compared against previous reports ensuring that the range was normal.22,23
Statistical analysis
The unpaired, 2-tailed Student t test was used to assess statistical significance between 2 data points throughout the study. GraphPad PRISM version 6.02 (GraphPad Software Inc., La Jolla, CA) was used for data analysis.
Results
HuFcγRIIIA-specific monovalent HSA fusion protein inhibits huIgG binding to huFcγRIIIA
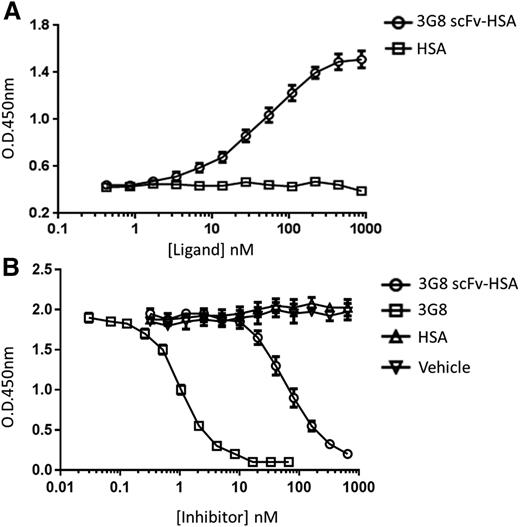
To investigate whether a monovalent 3G8 fused to albumin would retain its specificity, we generated the 3G8 scFv-HSA fusion protein and demonstrated its target specificity toward huFcγRIIIA (Figure 1A). Moreover, we examined its ability to inhibit the interaction between huIgG and huFcγRIIIA. As expected, 3G8 scFv-HSA was able to inhibit the binding of huIgG to huFcγRIIIA in a dose-dependent manner (Figure 1B). The inhibitor constants for 3G8 and 3G8 scFv-MSA are ∼1 nM and 40 nM, respectively (Figure 1B), demonstrating lowered binding efficiency of 3G8 scFv-MSA compared with its parent antibody 3G8 (Figure 1B), likely as a result of reduced multivalency and protein domain rearrangement.24-26 This may be important when the construct is developed for human ITP.
In vitro binding activity of 3G8 scFv-HSA for huFcγRIIIA. Binding of 3G8 scFv-HSA fusion protein to the soluble domain of huFcγRIIIA was assessed by ELISA. A high-binding plate was coated with recombinant huFcγRIIIA overnight. (A) To detect direct binding of 3G8 scFv-HSA to huFcγRIIIA, scFv-HSA or HSA (highest concentration: 870 nM) was added, and bound 3G8 scFv-HSA was detected by anti-HSA-HRP. n = 6 replicates; data representative of 3 independent experiments. (B) To assess the ability of 3G8 scFv-HSA to competitively inhibit huIgG binding to huFcγRIIIA, various concentrations of 3G8 scFv-HSA (highest concentration: 650 nM), HSA (highest concentration: 650 nM), 3G8 (highest concentration: 67 nM), or vehicle were added to wells containing 0.8 µg/mL huIgG. n = 4 replicates; data representative of 5 independent experiments. All data points represented as mean ± standard error of the mean.
In vitro binding activity of 3G8 scFv-HSA for huFcγRIIIA. Binding of 3G8 scFv-HSA fusion protein to the soluble domain of huFcγRIIIA was assessed by ELISA. A high-binding plate was coated with recombinant huFcγRIIIA overnight. (A) To detect direct binding of 3G8 scFv-HSA to huFcγRIIIA, scFv-HSA or HSA (highest concentration: 870 nM) was added, and bound 3G8 scFv-HSA was detected by anti-HSA-HRP. n = 6 replicates; data representative of 3 independent experiments. (B) To assess the ability of 3G8 scFv-HSA to competitively inhibit huIgG binding to huFcγRIIIA, various concentrations of 3G8 scFv-HSA (highest concentration: 650 nM), HSA (highest concentration: 650 nM), 3G8 (highest concentration: 67 nM), or vehicle were added to wells containing 0.8 µg/mL huIgG. n = 4 replicates; data representative of 5 independent experiments. All data points represented as mean ± standard error of the mean.
Monovalent 2.4G2 scFv-MSA fusion protein targets murine FcγRIII/IIB and exhibits favorable in vivo pharmacokinetics
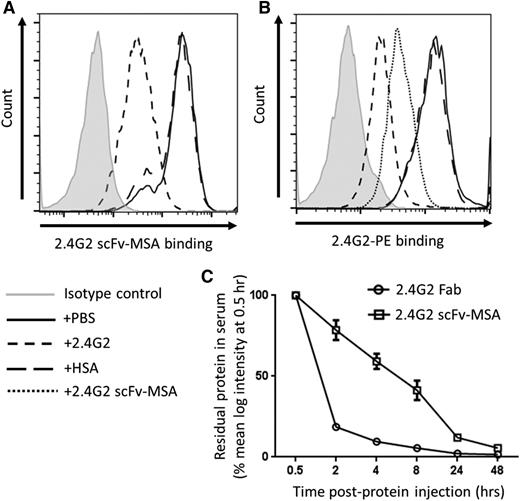
To investigate the in vivo efficacy and adverse event profile of monovalent targeting, we next generated 2.4G2 scFv-MSA fusion protein, the murine counterpart of 3G8 scFv-HSA that targets murine FcγRIII/IIB. The RAW264.7 macrophage-like cell line is known to express murine FcγRIII/IIB.20 The 2.4G2 scFv-MSA fusion protein was able to bind RAW264.7 cells (Figure 2A), and its binding activity could be inhibited by the parent 2.4G2 antibody, but not by HSA (Figure 2A). Conversely, the direct binding of 2.4G2 could be inhibited by 2.4G2 scFv-MSA and not by HSA (Figure 2B). Consistent with the reduced affinity exhibited by the human 3G8 scFv-HSA (Figure 1B), the parent 2.4G2 antibody displayed greater affinity than 2.4G2 scFv-MSA, evidenced by its superior ability to inhibit PE-labeled 2.4G2 binding to RAW264.7 cells (Figure 2B). After establishing 2.4G2 scFv-MSA target specificity, we next assessed its in vivo pharmacokinetics in comparison with the 2.4G2 Fab, another monovalent molecule. As expected, the large size and lasting property of MSA enabled 2.4G2 scFv-MSA to exhibit superior pharmacokinetics in vivo compared with 2.4G2 Fab (Figure 2C). Notably, ∼80% of 2.4G2 Fab was cleared within 2 hours of administration, whereas 2.4G2 scFv-MSA stayed higher than the Fab throughout all time points studied (Figure 2C). Previous findings show that the half-life of albumin in humans is ∼13 to 18 days,27,28 whereas that of mice is ∼1 day.29 The findings in this in vivo pharmacokinetics study are therefore consistent with previous reports and support the establishment that the half-life of albumin in mice is shorter than humans.
In vitro binding activity of 2.4G2 scFv-MSA for murine FcγRIII/IIB and in vivo pharmacokinetics. (A) RAW264.7 macrophage-like cell line, known to express FcγRIII and FcγRIIB, was stained with 0.11 µM 2.4G2 scFv-MSA (10 µg/mL) in the presence of vehicle control (PBS) or an equimolar amount of 2.4G2 or HSA (as competitive inhibitors). Residual bound 2.4G2 scFv-MSA was detected by anti-His-PE. Data representative of 4 independent experiments. (B) To analyze the ability of 2.4G2 scFv-MSA to inhibit PE-labeled 2.4G2 binding, RAW264.7 cells were stained with 0.013 µM (2 µg/mL) PE-labeled 2.4G2 in the presence of 0.11 µM (10 µg/mL) 2.4G2 scFv-MSA, HSA, 2.4G2, or PBS. Data representative of 5 independent experiments. (C) For in vivo pharmacokinetic analysis, mice were injected with 80 µg 2.4G2 scFv-MSA or ∼200 µg 2.4G2 Fab, and then bled after 0.5, 2, 4, 8, 24, and 48 hours. Serum samples were prepared and used to stain RAW264.7 cells; bound 2.4G2 scFv-MSA was detected by anti-His-PE, and bound 2.4G2 Fab in serum was detected by anti-rat IgG-κ chain-PE. The level of remaining serum protein was expressed as a percentage of mean log intensity at 0.5 hour. n = 6–8, from 3 independent experiments. Data are presented as mean ± standard error of the mean.
In vitro binding activity of 2.4G2 scFv-MSA for murine FcγRIII/IIB and in vivo pharmacokinetics. (A) RAW264.7 macrophage-like cell line, known to express FcγRIII and FcγRIIB, was stained with 0.11 µM 2.4G2 scFv-MSA (10 µg/mL) in the presence of vehicle control (PBS) or an equimolar amount of 2.4G2 or HSA (as competitive inhibitors). Residual bound 2.4G2 scFv-MSA was detected by anti-His-PE. Data representative of 4 independent experiments. (B) To analyze the ability of 2.4G2 scFv-MSA to inhibit PE-labeled 2.4G2 binding, RAW264.7 cells were stained with 0.013 µM (2 µg/mL) PE-labeled 2.4G2 in the presence of 0.11 µM (10 µg/mL) 2.4G2 scFv-MSA, HSA, 2.4G2, or PBS. Data representative of 5 independent experiments. (C) For in vivo pharmacokinetic analysis, mice were injected with 80 µg 2.4G2 scFv-MSA or ∼200 µg 2.4G2 Fab, and then bled after 0.5, 2, 4, 8, 24, and 48 hours. Serum samples were prepared and used to stain RAW264.7 cells; bound 2.4G2 scFv-MSA was detected by anti-His-PE, and bound 2.4G2 Fab in serum was detected by anti-rat IgG-κ chain-PE. The level of remaining serum protein was expressed as a percentage of mean log intensity at 0.5 hour. n = 6–8, from 3 independent experiments. Data are presented as mean ± standard error of the mean.
2.4G2 scFv-MSA inhibits FcγRIII- but not FcγRIV-mediated ITP
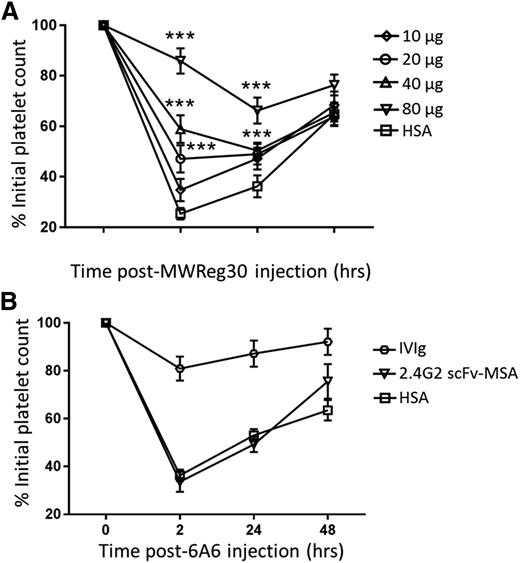
After establishing the target specificity and favorable pharmacokinetics, we next investigated the efficacy of 2.4G2 scFv-MSA in ITP amelioration. The antiplatelet antibody MWReg30 is known to mediate platelet clearance predominantly through FcγRIII,30,31 a target of 2.4G2 scFv-MSA. Pretreatment with 2.4G2 scFv-MSA for 2 hours before ITP induction by MWReg30 resulted in significantly higher platelet counts compared with the control (Figure 3A). Moreover, this ITP ameliorative effect was dose dependent (Figure 3A). Furthermore, the therapeutic effect of 2.4G2 scFv-MSA was maximal 2 hours post–antiplatelet antibody injection (ie, 4 hours after initial injection of 2.4G2 scFv-MSA) and declined 24 hours postinjection (Figure 3A). This diminutive trend over time correlates with the in vivo pharmacokinetics of 2.4G2 scFv-MSA (Figure 2C), consistent with the fact that MSA has a much shorter half-life as compared with larger primates. To further confirm the in vivo specificity of 2.4G2 scFv-MSA, we employed another antiplatelet antibody, 6A6 (of the murine IgG2a isotype), known to mediate platelet depletion via FcγRIV.32 We found that 80 µg 2.4G2 scFv-MSA, which significantly ameliorated MWReg30-induced ITP (Figure 3A), had no effect on 6A6-mediated platelet depletion (Figure 3B), demonstrating the expected in vivo specificity of 2.4G2 scFv-MSA.
In vivo efficacy of 2.4G2 scFv-MSA in ITP amelioration. (A) Mice were pretreated intravenously with 10, 20, 40, or 80 µg of 2.4G2 scFv-MSA or 56 µg HSA (equimolar amount as 80 µg 2.4G2 scFv-MSA) for 2 hours before ITP induction by administration of 2 µg antiplatelet antibody MWReg30. Mice were then bled after 2, 24, and 48 hours, and platelets were enumerated using a Z2 particle counter. ***P < .01 compared with HSA at each time point; n = 6–8, from 4 independent experiments. (B) Mice were pretreated with 25 mg IVIg (intraperitoneally), 80 µg 2.4G2 scFv-MSA, or 56 µg HSA for 2 hours before ITP induction by administration of 3 µg antiplatelet antibody 6A6. Mice were then bled after 2, 24, and 48 hours and platelets were enumerated using a Z2 particle counter. n = 6–7, from 4 independent experiments.
In vivo efficacy of 2.4G2 scFv-MSA in ITP amelioration. (A) Mice were pretreated intravenously with 10, 20, 40, or 80 µg of 2.4G2 scFv-MSA or 56 µg HSA (equimolar amount as 80 µg 2.4G2 scFv-MSA) for 2 hours before ITP induction by administration of 2 µg antiplatelet antibody MWReg30. Mice were then bled after 2, 24, and 48 hours, and platelets were enumerated using a Z2 particle counter. ***P < .01 compared with HSA at each time point; n = 6–8, from 4 independent experiments. (B) Mice were pretreated with 25 mg IVIg (intraperitoneally), 80 µg 2.4G2 scFv-MSA, or 56 µg HSA for 2 hours before ITP induction by administration of 3 µg antiplatelet antibody 6A6. Mice were then bled after 2, 24, and 48 hours and platelets were enumerated using a Z2 particle counter. n = 6–7, from 4 independent experiments.
The parent antibody 2.4G2, not 2.4G2 scFv-MSA, triggers body temperature decrease
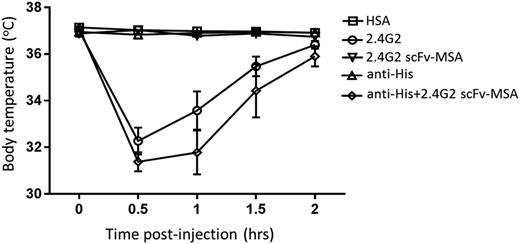
After establishing the in vivo efficacy of 2.4G2 scFv-MSA, we next examined whether 2.4G2 scFv-MSA induces in vivo adverse events. Consistent with previous reports,12,13 administration of 0.43 nmol (65 µg) 2.4G2 triggered a rapid drop in the body temperature of mice, which was recovered by 2 hours (Figure 4). A similar decrease in body temperature was absent when mice were treated with 2.4G2 scFv-MSA or HSA (Figure 4). To investigate whether reversing the monovalency of 2.4G2 scFv-MSA would recapitulate the drop in body temperature, we used a monoclonal anti-His antibody to cross-link 2.4G2 scFv-MSA. Treatment with a cross-linked preparation of 2.4G2 scFv-MSA induced a similar drop in body temperature as compared with the parent 2.4G2 antibody (Figure 4).
Changes in body temperature induced by antibody 2.4G2 and 2.4G2 scFv-MSA. Mice were treated with 0.43 nmol (65 µg) 2.4G2 or equimolar amounts of 2.4G2 scFv-MSA or HSA. Cross-linked 2.4G2 scFv-MSA was prepared by mixing 0.43 nmol 2.4G2 scFv-MSA and half-molar (0.22 nmol) anti-His mAb for 30 minutes at room temperature. Body (rectal) temperature was measured 0.5, 1, 1.5, and 2 hours after treatment by a thermometer. n = 6–9, from 3 independent experiments.
Changes in body temperature induced by antibody 2.4G2 and 2.4G2 scFv-MSA. Mice were treated with 0.43 nmol (65 µg) 2.4G2 or equimolar amounts of 2.4G2 scFv-MSA or HSA. Cross-linked 2.4G2 scFv-MSA was prepared by mixing 0.43 nmol 2.4G2 scFv-MSA and half-molar (0.22 nmol) anti-His mAb for 30 minutes at room temperature. Body (rectal) temperature was measured 0.5, 1, 1.5, and 2 hours after treatment by a thermometer. n = 6–9, from 3 independent experiments.
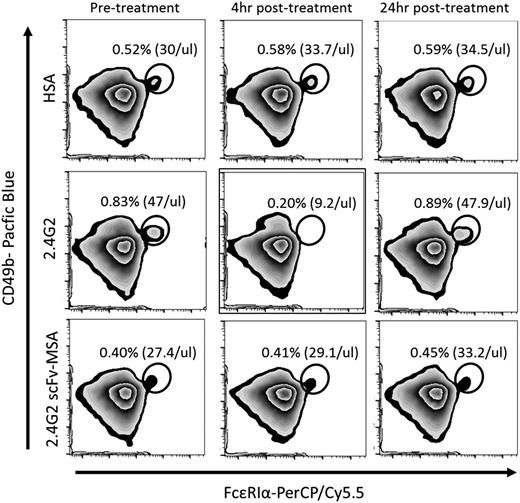
Antibody 2.4G2–induced basophil activation and depletion is absent in response to 2.4G2 scFv-MSA
In addition to changes in body temperature, we examined the basophil activation–related marker CD200R3. A recent report demonstrated that 2.4G2-induced anaphylaxis significantly reduced basophil expression of CD200R3, an activating cell surface receptor.13 CD200R3 was expressed on basophils (Figure 5A-B). The administration of 0.43 nmol (65 µg) 2.4G2 rapidly reduced the ability to detect CD200R3 on basophils, which partially recovered after 24 hours (Figure 5C). In contrast, neither 2.4G2 scFv-MSA nor HSA significantly modulated CD200R3 levels. In addition to CD200R3 expression, we observed a transient basophil depletion in response to 2.4G2 administration, which was also largely recovered after 24 hours (Figure 6). In contrast, both 2.4G2 scFv-MSA and HSA had no significant effect on blood basophil levels (Figure 6).
Basophil activation induced by antibody 2.4G2 and 2.4G2 scFv-MSA. (A) To analyze CD200R3 levels on basophils, RBCs in peripheral blood were lysed by ammonium chloride buffer before staining with anti-CD49b-Pacific Blue, anti-FcεRIα-PerCP/Cy5.5, and anti-CD200R3-FITC. The population within gate P1 represents PBMCs (left) and was further gated based on CD49b and FcεRIα expression levels. The population within P2 (middle) represents basophils (P2 shown in FCS and SSC plot, right), evidenced by expression of CD200R3 (B). (C) Mice were bled before treatment and 4 and 24 hours after administration of 0.43 nmol (65 µg) 2.4G2 or equimolar amounts of 2.4G2 scFv-MSA or HSA. Samples were stained with anti-CD49b-Pacific Blue, anti-FcεRIα-PerCP/Cy5.5, and anti-CD200R3-FITC. All samples were analyzed by MACS Quant. Data were analyzed by Flowjo V10 software. Dot plots and histograms representative of 6 to 7 mice per group from 4 independent experiments.
Basophil activation induced by antibody 2.4G2 and 2.4G2 scFv-MSA. (A) To analyze CD200R3 levels on basophils, RBCs in peripheral blood were lysed by ammonium chloride buffer before staining with anti-CD49b-Pacific Blue, anti-FcεRIα-PerCP/Cy5.5, and anti-CD200R3-FITC. The population within gate P1 represents PBMCs (left) and was further gated based on CD49b and FcεRIα expression levels. The population within P2 (middle) represents basophils (P2 shown in FCS and SSC plot, right), evidenced by expression of CD200R3 (B). (C) Mice were bled before treatment and 4 and 24 hours after administration of 0.43 nmol (65 µg) 2.4G2 or equimolar amounts of 2.4G2 scFv-MSA or HSA. Samples were stained with anti-CD49b-Pacific Blue, anti-FcεRIα-PerCP/Cy5.5, and anti-CD200R3-FITC. All samples were analyzed by MACS Quant. Data were analyzed by Flowjo V10 software. Dot plots and histograms representative of 6 to 7 mice per group from 4 independent experiments.
Transient basophil depletion induced by antibody 2.4G2 and 2.4G2 scFv-MSA. Mice were bled before treatment and 4 and 24 hours after treatment with 0.43 nmol (65 µg) 2.4G2 or equimolar amounts of 2.4G2 scFv-MSA or HSA. Ammonium chloride buffer was used to lyse RBCs before PMBCs were stained with anti-CD49b-Pacific Blue, anti-FcεRIα-PerCP/Cy5.5, and anti-CD200R3-FITC. Stained samples were analyzed by MACS Quant, and data were analyzed by Flowjo V10 software. Basophils were identified as CD49 dim, FcεRIα positive, and CD200R3 positive. A round gate is used to mark basophil population. The frequency represents the percentage of basophils within the whole PBMC population shown as P1 in Figure 5A. Basophil concentrations (per microliter blood) represent in vivo concentrations. Histograms are representative of 6 to 7 mice per group from 4 independent experiments.
Transient basophil depletion induced by antibody 2.4G2 and 2.4G2 scFv-MSA. Mice were bled before treatment and 4 and 24 hours after treatment with 0.43 nmol (65 µg) 2.4G2 or equimolar amounts of 2.4G2 scFv-MSA or HSA. Ammonium chloride buffer was used to lyse RBCs before PMBCs were stained with anti-CD49b-Pacific Blue, anti-FcεRIα-PerCP/Cy5.5, and anti-CD200R3-FITC. Stained samples were analyzed by MACS Quant, and data were analyzed by Flowjo V10 software. Basophils were identified as CD49 dim, FcεRIα positive, and CD200R3 positive. A round gate is used to mark basophil population. The frequency represents the percentage of basophils within the whole PBMC population shown as P1 in Figure 5A. Basophil concentrations (per microliter blood) represent in vivo concentrations. Histograms are representative of 6 to 7 mice per group from 4 independent experiments.
Discussion
Fc receptor blockade has long been considered a viable strategy to treat antibody-mediated platelet destruction.9,10 Some existing ITP therapeutics, such as anti-D and IVIg, have been speculated to include a level of Fc receptor blockade in their modes of action.33-35 The huFcγRIIIA-specific mAb 3G8 was first described in 19827 and shown to improve ITP in refractory patients. The effective reversal of the low platelet count by the first anti-huFcγRIIIA antibody, 3G8, suggested the possibility of superseding current plasma-derived therapeutics with a monoclonal substitute.8,10 However, the clinical adverse events encountered during the pilot trials forestalled further development.8,11 Although the exact cause of these adverse events remains unclear, a main potential mechanism involved the multivalent cross-linking of the activatory FcγRIIIA, mediated by the antigen-binding domain and Fc domain of the antibody. Based on this theory, a second-generation anti-huFcγRIIIA antibody, GMA161, engineered to lack Fc-mediated FcγR engagement, had been developed.15 However, GMA161 failed to arrest the adverse events in refractory ITP patients, pointing out the genesis of these adverse events by some other attribute of the therapeutic antibody. We have speculated in the current work that this adverse event profile might be contributed to by the bivalent antigen-binding domain of anti-FcγR antibodies.11
Activating FcγRs can normally be cross-linked by the IgG Fc, typically by the formation of immune complexes, to initiate an immune response.6 Such coordinated FcγR cross-linking is crucial for antibody-mediated immune function.6 However, uncontrolled cross-linking, as occurs upon the injection of anti-FcγR antibodies, could lead to undesired adverse events, demonstrated by the trials of 3G8 and GMA161.8,11 Such anti-FcγR antibody–induced anaphylaxis is reminiscent of systemic inflammation triggered by certain pathological superantigens.36 To overcome the multivalency intrinsic to an anti-FcγR antibody, we developed a monovalent approach in an attempt to circumvent the adverse events while retaining therapeutic efficacy. We first generated a fusion protein (3G8 scFv-HSA) composed of a single huFcγRIIIA-binding domain of 3G8 fused to HSA, which retained the ability to bind huFcγRIIIA and inhibit IgG-huFcγRIIIA interaction.
Next, to investigate the in vivo feasibility of such a monovalent approach, we generated a fusion protein (2.4G2 scFv-MSA) composed of a single FcγRIII-binding domain of 2.4G2 fused to MSA and demonstrated its therapeutic efficacy in a passive murine ITP model. Moreover, we show that 2.4G2 scFv-MSA successfully overcame the 2.4G2 antibody–induced body temperature decrease, a common measure of anaphylaxis.12,13,37 Importantly, we also demonstrated that by cross-linking 2.4G2 scFv-MSA, the decrease in body temperature was recapitulated, further supporting a major role of multivalent cross-linking in causing adverse events. However, the addition of the anti-His antibody could potentially generate micro–immune complexes with not only bivalent anti-FcγR reactivity but also an intact Fc domain contributed by the anti-His antibody. In addition to body temperature, 2.4G2 scFv-MSA lacked the ability to activate basophils demonstrated by its parent 2.4G2 antibody. Basophils are known to be pivotal for IgG-induced anaphylaxis,38 and the basophilic surface receptor CD200R3 has recently been demonstrated to be a marker for anti-FcγR antibody–mediated anaphylaxis.13,38 We confirmed the finding that the 2.4G2-mediated anaphylactic response significantly lowered CD200R3 levels on basophils, an effect absent in response to 2.4G2 scFv-MSA treatment. In addition to the decreased CD200R3 level, we observed a transient basophil depletion in response to 2.4G2 treatment, but not 2.4G2 scFv-MSA. Murine basophils are known to express significant levels of FcγRIII39 and thus would be a target for 2.4G2-mediated depletion. This transient depletion is consistent with the anti-huFcγRIIIA GMA161-induced granulocyte depletion in the humanized mouse model.15
The 2.4G2 scFv-MSA exhibited superior pharmacokinetics compared with the monovalent Fab fragment, likely as a result of its larger size and the extended half-life of albumin. Indeed, in recent years, significant progress has been made to prolong the half-life of protein-based therapeutics, culminating in the approval of several clinical products.40 Some notable strategies include increasing the size of the protein41,42 or conferring binding affinity to the FcRn,43,44 a receptor conferring extended half-life of IgG and albumin.45 Albumin-coupled therapeutics have recently entered the list of approved medicines,46,47 further supporting the feasibility of this albumin fusion protein. Although 2.4G2 scFv-MSA exhibited significantly improved pharmacokinetics compared with its Fab counterpart, ∼90% was cleared within the first 24 hours, raising the issue of short-lasting in vivo efficacy. Previous studies have conclusively established that the in vivo longevity of albumin is directly proportional to the size of the animal, with mice having the shortest half-life.27,28,48-50 This shorter half-life of albumin in mice prevented us from establishing a therapeutic ITP mouse model, as such a model requires the treatment to typically stay in circulation for 2 days to enable detection of the therapeutic effects.51 Thus, independent models involving larger animals could help investigate its efficacy in active ITP or in other FcγR-implicated diseases.
In summary, we demonstrate in this work that monovalent targeting of FcγRs is able to ameliorate antibody-mediated ITP while circumventing cross-linking–induced adverse events normally triggered by the FcγR-specific antibody. Fusion of the monovalent antibody domain to albumin has allowed the fusion protein to exhibit favorable pharmacokinetics compared with its Fab counterpart. We speculate that this approach may not only be useful in ITP but may also be beneficial in other FcγR-mediated diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the St. Michael’s Hospital Vivarium staff for their help with animal care.
X.Y. is funded by a postdoctoral fellowship from the Canadian Blood Services (CBS). This work was supported by peer-reviewed operating grants from CBS/Canadian Institutes of Health Research Partnership (FRN6897) (A.H.L.) and CBS Intramural Operating Grant program (2013-IG-AL00545) (A.H.L.). Resources for both programs are provided by Health Canada, a division of the federal government of Canada.
The views expressed herein do not necessarily represent the views of the federal government of Canada.
Authorship
Contribution: X.Y., W.P.S., and A.H.L. designed the experiments; X.Y., M.M., V.B., and J.P., performed the experiments; X.Y., W.P.S., A.H.L., and J.P. analyzed the data; and X.Y. and A.H.L. wrote the manuscript.
Conflict-of-interest disclosure: A.H.L. has patents on the use of mAb’s to treat ITP. The remaining authors declare no competing financial interests.
Correspondence: Alan. H. Lazarus, The Keenan Research Centre, St. Michael’s Hospital, 30 Bond St, Toronto, ON, Canada M5B 1W8; e-mail: lazarusa@smh.ca.