Key Points
Activation of ATM kinase modulates neutrophil functions and is dependent on the oxidative burst.
Neutrophils from ataxia telangiectasia patients overproduce inflammatory cytokines and have a prolonged lifespan.
Abstract
Neutrophils play an essential role in the initial stages of inflammation by balancing pro- and antiinflammatory signals. Among these signals are the production of proinflammatory cytokines and the timely initiation of antiinflammatory cell death via constitutive apoptosis. Here we identify ataxia-telangiectasia mutated (ATM) kinase as a modulator of these neutrophil functions. Ataxia-telangiectasia (AT) is a pleiotropic multisystem disorder caused by mutations in the gene-encoding ATM, a master regulator of the DNA damage response. In addition to progressive neurodegeneration and high rates of cancer, AT patients have numerous symptoms that can be linked to chronic inflammation. We report that neutrophils isolated from patients with AT overproduce proinflammatory cytokines and have a prolonged lifespan compared with healthy controls. This effect is partly mediated by increases in activation of p38 MAP kinase. Furthermore, we show that the oxidative burst, catalyzed by nicotinamide adenine dinucleotide phosphate oxidase, can activate ATM in neutrophils. Finally, activation of ATM and DNA damage signaling suppress cytokine production and can abrogate the overproduction of IL-8 in ROS-deficient cells. This reveals a novel mechanism for the regulation of cytokine production and apoptosis, establishing DNA damage as a downstream mediator of immune regulation by reactive oxygen species. We propose that deficiencies in the DNA damage response, like deficiencies in the oxidative burst seen in chronic granulomatous disease, could lead to pathologic inflammation.
Introduction
The inflammatory response is tightly regulated to control its duration and magnitude and to limit the extent of collateral damage to host tissues. Improper regulation can lead to exaggerated inflammatory responses and pathology. Chronic inflammation is destructive to tissues and is a pathologic component of many diverse diseases including cancer, cardiovascular disease, insulin-resistant diabetes, and neurological diseases.
Ataxia-telangiectasia (AT) is a pleiotropic, recessive genetic disorder that occurs in an estimated one in 40 000 to 100 000 live births. AT affects multiple-organ systems and is characterized by progressive neurodegeneration, radiosensitivity, sterility, growth defects, variable immunodeficiency, and increased susceptibility to cancer.1 Until now, immune dysfunction in AT has been characterized by improper B- and T-cell maturation,2-5 immunoglobulin deficiencies, and impaired antibody responses observed in about two-thirds of patients.6,7 Patients often have recurring respiratory tract infections and chronic interstitial inflammatory lung disease, although opportunistic infections are uncommon.8,9 Many symptoms in AT patients, including increased incidence of autoimmunity,10 oxidative stress,11 cardiovascular disease, insulin resistance, and elevated serum levels of inflammatory cytokines,12,13 suggest that they also suffer from pathologic inflammation. Furthermore, there are several reports on patients presenting with chronic cutaneous granulomas and pulmonary inflammation without apparent underlying infections.14-17 Although the immune defects in the adaptive arm of the immune system associated with this disease have been well studied, the effects on the innate immune system remain poorly understood.
Biallelic mutations in ataxia-telangiectasia mutated (ATM) are the underlying cause of AT. ATM is a large serine/threonine protein kinase involved in various cellular processes.18 ATM is an important sensor of DNA damage, along with ATR (AT and RAD3-related) and DNA-dependent protein kinase (DNA-PK). In response to genotoxic insults, ATM is phosphorylated and then initiates and coordinates the DNA damage response. DNA damage signaling arrests the cell cycle until the lesions are resolved or, if the damage is beyond repair, can initiate apoptosis. ATM and DNA-PK are activated in response to double-strand breaks, and ATR by single-strand breaks and incompletely replicated DNA.19 Substrates of these kinases include histone variant H2A.X (a marker of DNA damage known as γ-H2A.X when phosphorylated), cell-cycle regulators Chk1 and Chk2, and the apoptosis regulator p53.18 The role of ATM in regulating cellular processes is not limited to DNA damage; it also coordinates responses to other forms of stress to restore homeostasis.20 Notably, ATM was recently shown to be activated by oxidative stress21 and was suggested to be involved in redox regulation.22
Neutrophils are the first cells recruited in large numbers to sites of inflammation where, in addition to antimicrobial activity, they exert both pro- and antiinflammatory functions to balance the immune response.23 Recognition of microbial components via pattern recognition receptors stimulates neutrophils by activating NF-κB and initiating mitogen-activated protein kinase (MAPK) signaling cascades. These pathways result in the production and secretion of proinflammatory cytokines, including interleukin 8 (IL-8) and macrophage inflammatory protein-1α (MIP-1α), which recruit and activate more immune cells. Activated neutrophils additionally mount an oxidative burst, producing large amounts of reactive oxygen species (ROS) via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex. ROS are highly volatile molecules that damage many cellular components of microbes as well as the host, and additionally function as signaling molecules.24
Neutrophils contribute to the regulation of inflammation through multiple processes. Their lifespan is tightly regulated to limit their proinflammatory functions. In addition, apoptosis of the large population of infiltrating neutrophils provides a strong antiinflammatory signal via efferocytosis, the removal of apoptotic cells via phagocytosis by other immune cells. Efferocytosis dampens proinflammatory cytokine production and initiates the resolution of inflammation.25,26 In addition to antimicrobial activity, the oxidative burst in phagocytes contributes to regulation of both cytokine production and apoptosis, thus playing an important role in balancing inflammation.27 Although the exact mechanisms are still unknown, deficiencies in the neutrophil oxidative burst result in chronic granulomatous disease, characterized by impaired host defenses and hyperinflammation.
Here we show that neutrophils from AT patients overproduce proinflammatory cytokines and have a longer lifespan than those from healthy controls. We demonstrate that the activation of ATM and the DNA damage response in neutrophils normally plays a role in regulating cytokine production and apoptosis in a manner dependent on NADPH oxidase activity. Furthermore, we describe a similar effect of ATM inhibition on monocyte cytokine production, suggesting the existence of a common regulatory mechanism in myeloid cells. The absence of ATM activity, as with the oxidative burst, could thus disrupt immune regulation and potentially tip the scales in the direction of chronic inflammation.
Methods
Donor consent
Our study was conducted in accordance with the Helsinki Declaration. Blood samples were collected with approval from the ethical committees of each institution. Informed consent was provided by all patients, or by their parents, in the case of children. At the time of blood donation, participants displayed no signs of infection or malignancy. Patients were receiving no treatments or medications apart from regular IV immunoglobulin therapy.
Neutrophil and monocyte isolation
Human neutrophils were isolated by centrifuging heparinized venous blood over Histopaque 1119 (Sigma-Aldrich) and subsequently over a discontinuous Percoll (Amersham Biosciences) gradient as previously described.28 Peripheral blood monocytes were isolated by magnetic-activated cell sorting selection using magnetically-labeled CD14 microbeads (Miltenyi Biotec). Cell preparations were at least 95% neutrophils or monocytes. Cells were cultured in RPMI 1640 (Gibco) supplemented with 100 U/mL penicillin and streptomycin, 292 µg/mL l-glutamine (Gibco), and 10% fetal calf serum (Sigma).
Cytokine production assays
Cells were seeded in 96-well plates with indicated inhibitors or dimethyl sulfoxide control and stimulated for 18 hours with 100 ng/mL lipopolysaccharide (LPS from Salmonella typhimurium [TLR grade], Enzo Life Sciences; 10 µg/mL opsonized Zymosan [Sigma-Aldrich], MOI 100 heat-killed Listeria monocytogenes [HKLM, InvivoGen], or 1 µg/mL flagellin [from S typhimurium, InvivoGen]). Zymosan was opsonized with human plasma from multiple donors. Inhibitors of ATM (KU-55933 and KU-60019, 10 µM unless otherwise stated), ATR (VE-821, 1 µM), DNA-PK (NU-7441, 1 µM), Chk2 (PV-1019, 10 µM), and p53 (Pifithrin-µ, 1 µM) were obtained from Calbiochem. Cisplatin and etoposide were obtained from Calbiochem and Sigma-Aldrich, respectively. P38 inhibitor (SB203580, 5 µM) and ERK inhibitor (PD98059, 5 µM) were obtained from New England Biolabs. Cytokine concentrations were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D systems DuoSet ELISAs).
Annexin V viability assay
Neutrophil viability was determined using the PE-Annexin V apoptosis kit (BD Biosciences). Briefly, neutrophils were resuspended at the indicated time points, washed in Annexin V binding buffer, stained with PE-Annexin V and vital dye propidium iodide (PI), and then analyzed by flow cytometry. Annexin V/PI double-negative neutrophils were defined as viable (supplemental Figure 3, available on the Blood Web site). We found that we typically recover >90% of the cells after 18 hours. At least 10 000 cells were measured per sample.
Quantitative real-time PCR
RNA was isolated with the RNeasy mini kit (Qiagen). cDNA was made using a high-capacity RNA-to-cDNA kit (Applied Biosystems) according to manufacturer’s protocol. Quantitative real-time polymerase chain reaction (qPCR) was performed on StepOnePlus Real-Time PCR System with 2x Fast SYBR Green master mix (Applied Biosystems) according to protocol with cDNA made from 10 ng of RNA per reaction. Previously verified primers29 were used for IL-8 F 5′-CTGGCCGTGGCTCTCTTG-3′ and R 5′-CCTTGGCAAAACTGCACCTT-3′, MIP-1α F 5′-AGCTGACTACTTTGAGACGAGCA-3′ and R 5′-CGGCTTCGCTTGGTTAGGA-3′, and housekeeping gene β2-microglobulin F 5′-CTCCGTGGCCTTAGCTGTG-3′ and R 5′-TTTGGAGTACGCTGGATAGCCT-3′. Data were analyzed using StepOne software and expressed as relative amount of IL-8 or MIP-α product as determined from pooled standard curve divided by the relative amount of β2- microglobulin product at each time point.
Intracellular IL-8 staining
Neutrophils were incubated with 3 µg/mL Brefeldin A and stimulated with LPS as described before. At indicated time points, neutrophils were resuspended and fixed with 2% paraformaldehyde, washed and resuspended in phosphate-buffered saline 1% bovine serum albumin, and stored at 4°C. Cells were permeabilized with saponin, washed, and stained with PE-mouse anti-human IL-8 or isotype control antibody (BD Pharmingen), and analyzed by flow cytometry on a MACSQuant. At least 10 000 cells were measured per sample.
Western blot
Lysates were made from 2 × 106 cells resuspended in 100 µL of Laemmli’s buffer supplemented with protease inhibitor cocktail (Sigma), Halt! Phosphatase inhibitor (Fischer Scientific), and 2 µM neutrophil elastase inhibitor V (Calbiochem). Protein lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by analysis by Western immunoblotting. Anti–phospho-histone H2A.X (Ser139) was from Millipore. All other antibodies used were obtained from Cell Signaling Technologies.
Immunofluorescence
Neutrophils stimulated with 100 nM phorbol 12-myristate 13-acetate (PMA) on glass coverslips were fixed with 2% paraformaldehyde 90 minutes after activation, permeabilized 5 minutes in ice cold acetone, and then blocked at 37°C for 1 hour in 1% bovine serum albumin, 5% normal donkey serum, and 15% cold-water fish gelatin (Sigma-Aldrich). Samples were stained in blocking buffer with 2 µg/mL mouse anti-human phospho-ATM (Ser 1981) (Thermo Scientific), followed by secondary antibody goat-anti mouse IgG conjugated to Alexa Fluor 568 (Invitrogen). Coverslips were mounted on ProLong Gold antifade mountant with DNA dye 4,6 diamidino-2-phenylindole (Thermo Fisher Scientific). Images were taken with a Leica SP8 confocal microscope.
Results
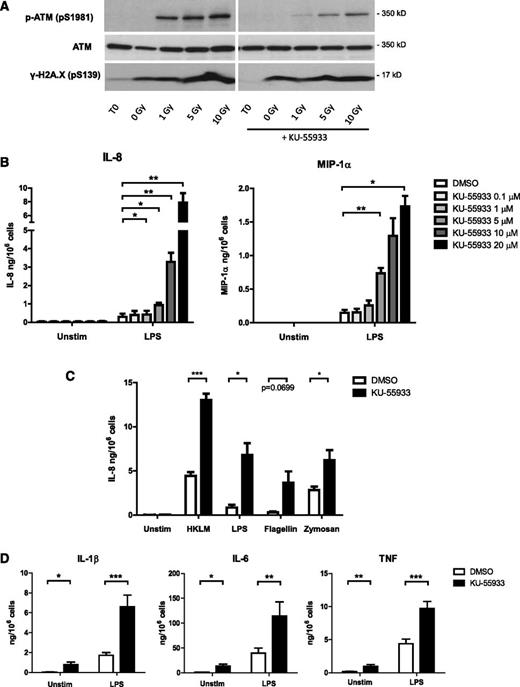
We assayed the effects of ATM activity on neutrophil functions by incubating cells with various inflammatory stimuli in the presence of KU-55933, a highly-specific small-molecule inhibitor of ATM.30 Neutrophils express functional ATM, which is phosphorylated in response to irradiation-induced DNA damage, and this activation is abrogated in the presence of KU-55933 (Figure 1A). We found that this inhibitor caused a strong dose-dependent increase in the secretion of IL-8 and MIP-1α in response to LPS, a component of bacterial outer membranes (Figure 1B). The specificity of this effect was confirmed using a second inhibitor of ATM, KU-60019 (supplemental Figure 1). We observed an increase in cytokine production in the presence of KU-55933 not only in response to LPS stimulation, but also in response to opsonized zymosan, flagellin, and heat-killed L monocytogenes (Figure 1C). An increase in the production of IL-6 in response to LPS and heat-killed Listeria was similarly observed (supplemental Figure 2). Moreover, ATM inhibition in peripheral blood monocytes significantly increased the production of IL-1β, IL-6, and tumor necrosis factor in response to LPS stimulation (Figure 1D).
Inhibition of ATM activity in neutrophils increases proinflammatory cytokine production. (A) Western blot of ATM, phosphorylated ATM, and γ-H2A.X in lysates from neutrophils exposed to γ irradiation and incubated for 1 hour with and without 10 µM ATM inhibitor KU-55933. (B) Neutrophil IL-8 (n = 4) and MIP-1α (n = 3) production in response to LPS stimulation in the presence of increasing KU-55933 concentration. Isolated peripheral blood neutrophils from healthy donors were incubated with ATM inhibitor and stimulated with LPS for 18 hours. Cytokine concentrations in supernatants were measured by ELISA. (C) Neutrophil IL-8 production in response to 18-hour stimulation with flagellin (1 μg/mL), LPS (100 ng/mL), opsonized zymosan (10 μg/mL), or heat-killed L monocytogenes (HKLM, MOI = 100) in the presence of KU-55933 (10 μM) (n = 3-4). (D) Monocyte cytokine production in response to LPS stimulation in the presence of KU-55933. Isolated peripheral blood monocytes from healthy donors (n = 10) were incubated with KU-55933 (10 μM) and stimulated for 18 hours with LPS. (B-D) Data are presented as the mean ± standard error of the mean (SEM) of compiled experiments. Asterisks indicate significant increases: *P < .05, **P < .01, ***P < .001 by paired Student t test.
Inhibition of ATM activity in neutrophils increases proinflammatory cytokine production. (A) Western blot of ATM, phosphorylated ATM, and γ-H2A.X in lysates from neutrophils exposed to γ irradiation and incubated for 1 hour with and without 10 µM ATM inhibitor KU-55933. (B) Neutrophil IL-8 (n = 4) and MIP-1α (n = 3) production in response to LPS stimulation in the presence of increasing KU-55933 concentration. Isolated peripheral blood neutrophils from healthy donors were incubated with ATM inhibitor and stimulated with LPS for 18 hours. Cytokine concentrations in supernatants were measured by ELISA. (C) Neutrophil IL-8 production in response to 18-hour stimulation with flagellin (1 μg/mL), LPS (100 ng/mL), opsonized zymosan (10 μg/mL), or heat-killed L monocytogenes (HKLM, MOI = 100) in the presence of KU-55933 (10 μM) (n = 3-4). (D) Monocyte cytokine production in response to LPS stimulation in the presence of KU-55933. Isolated peripheral blood monocytes from healthy donors (n = 10) were incubated with KU-55933 (10 μM) and stimulated for 18 hours with LPS. (B-D) Data are presented as the mean ± standard error of the mean (SEM) of compiled experiments. Asterisks indicate significant increases: *P < .05, **P < .01, ***P < .001 by paired Student t test.
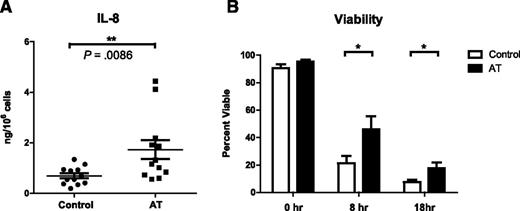
To determine whether genetic deficiencies in ATM affect neutrophil cytokine production in patients, we measured IL-8 production from primary peripheral blood neutrophils isolated from 11 different patients with AT (mean age, 16 years). One of these patients participated twice on separate occasions (n = 12). All patients were free of infections and malignancy at the time of blood donation. AT diagnoses were confirmed by ATM gene sequencing (supplemental Table 1). Neutrophils isolated from 12 sex-matched healthy donors were used as controls (mean age, 28 years; n = 12). Neutrophils were isolated and experiments performed with one AT patient and one control concurrently. Neutrophils from patients with AT produced significantly more IL-8 (mean, 1.73 ng/106 cells) in response to LPS than healthy controls (mean, 0.69 ng/106 cells) (Figure 2A). These data, complemented by our experiments with ATM inhibitors, show that cytokine production is increased in the absence of ATM activity.
Primary neutrophils isolated from AT patients produce more IL-8 and have a prolonged lifespan. (A) IL-8 production from LPS-stimulated peripheral blood neutrophils isolated from AT patients (mean = 1.73 ng, n = 12) or healthy controls (mean = 0.69 ng, n = 12) 18 hours after stimulation (P = .0086, Mann-Whitney U test). IL-8 concentrations in supernatants were measured by ELISA and normalized to cell number. Data are plotted as mean ± SEM. (B) 18-hour viability curve of primary neutrophils isolated concurrently from AT patients (n = 5-7) and healthy controls (n = 6-8). At the indicated time points, neutrophils were collected and stained with PE-Annexin V and PI for viability. 10 000 cells per condition were analyzed by flow cytometry. Annexin V/PI-double-negative cells were defined as viable. Data are plotted as mean ± SEM. Asterisks indicate significant increases: *P < .05, **P < .01, ***P < .001 by paired Student t test.
Primary neutrophils isolated from AT patients produce more IL-8 and have a prolonged lifespan. (A) IL-8 production from LPS-stimulated peripheral blood neutrophils isolated from AT patients (mean = 1.73 ng, n = 12) or healthy controls (mean = 0.69 ng, n = 12) 18 hours after stimulation (P = .0086, Mann-Whitney U test). IL-8 concentrations in supernatants were measured by ELISA and normalized to cell number. Data are plotted as mean ± SEM. (B) 18-hour viability curve of primary neutrophils isolated concurrently from AT patients (n = 5-7) and healthy controls (n = 6-8). At the indicated time points, neutrophils were collected and stained with PE-Annexin V and PI for viability. 10 000 cells per condition were analyzed by flow cytometry. Annexin V/PI-double-negative cells were defined as viable. Data are plotted as mean ± SEM. Asterisks indicate significant increases: *P < .05, **P < .01, ***P < .001 by paired Student t test.
Because neutrophil apoptosis is regulated by p53, which can be induced by ATM activation,31 we asked whether ATM deficiency affects neutrophil lifespan. Cell viability was assayed by flow cytometry over an 18-hour time course by staining with PE-labeled Annexin V (which binds exposed phosphatidylserine on the surface of apoptotic cells) and propidium iodide (PI, a vital DNA dye), at the indicated time points. We found that neutrophils from AT patients lived significantly longer than controls (Figure 2B). We confirmed this result by measuring viability with the Sub-G1 assay (supplemental Figure 4). Taken together, these data show that primary neutrophils from AT patients overproduce inflammatory cytokines and delay constitutive apoptosis.
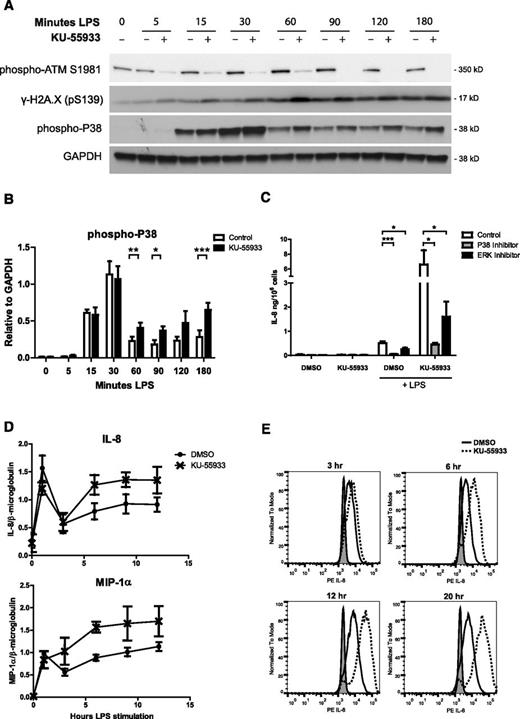
In response to LPS stimulation, MAP kinases are activated by phosphorylation, whereas IκBα, an inhibitor of the transcription factor NF-κB, is degraded. To determine whether ATM regulates the activation or duration of these signaling pathways, we measured their activation in response to LPS stimulation by Western blot. In a 3-hour time course of LPS stimulation, we observed phosphorylation of ATM and histone H2A.X. Phosphorylation of ATM, but not H2A.X, in response to LPS was abrogated by the addition of KU-55933, where p38 phosphorylation was increased (Figure 3A-B). We observed increased ERK phosphorylation and degradation of IκBα, but only at later time points (supplemental Figure 5). We then used inhibitors of p38 and ERK to determine whether IL-8 overproduction in the absence of ATM activity is dependent on these MAP kinases. P38 inhibition blocked IL-8 production in controls and abrogated the overproduction of IL-8 in response to ATM inhibition (Figure 3C).
ATM inhibition increases p38 activation as well as cytokine transcription and production in stimulated neutrophils. (A) Western blot analysis of phosphorylation of ATM, histone H2A.X, and p38 in neutrophil lysates over a 180-minute time course of LPS stimulation with and without 10 µM KU-55933, representative blot of 4 experiments. (B) Relative levels of phospho-p38 quantified by densitometry in ImageJ software. Data are expressed as signal relative to the loading control GAPDH (n = 4). (C) Effect of p38 and ERK inhibitors on neutrophil IL-8 production in the presence of KU-55933 in response to LPS stimulation. IL-8 concentration in supernatants was measured after 18 hours of stimulation (n = 5) (D). Quantitative real-time PCR analysis of relative IL-8 and MIP-1α transcripts in cDNA from neutrophils stimulated with LPS with and without KU-55933. mRNA was isolated from LPS-stimulated neutrophils at the indicated time points, converted to cDNA, and analyzed by qPCR in triplicate (n = 3). Data are expressed as the relative amount of cytokine transcript divided by the relative amount of housekeeping gene β2-microglobulin (n = 3). Relative transcript amounts were calculated using a standard curve made from serial dilutions of a pooled sample. Data were analyzed using StepOnePlus software. (D) Intracellular IL-8 staining of LPS-stimulated neutrophils in the presence of KU-55933. Neutrophils were incubated with Brefeldin A to block secretion and stimulated with LPS with or without ATM inhibitor. At the indicated time points, cells were fixed and stained with an anti-IL8 antibody and analyzed by flow cytometry. At least 10 000 cells were analyzed per sample. Gray fill represents unstimulated neutrophils, the solid line represents LPS-stimulated dimethyl sulfoxide control, and the dashed line represents LPS-stimulated with 10 µM KU-55933. (B-D) Data are presented as the mean ± SEM of compiled experiments. Asterisks indicate significance: *P < .05, **P < .01, ***P < .001 by paired Student t test.
ATM inhibition increases p38 activation as well as cytokine transcription and production in stimulated neutrophils. (A) Western blot analysis of phosphorylation of ATM, histone H2A.X, and p38 in neutrophil lysates over a 180-minute time course of LPS stimulation with and without 10 µM KU-55933, representative blot of 4 experiments. (B) Relative levels of phospho-p38 quantified by densitometry in ImageJ software. Data are expressed as signal relative to the loading control GAPDH (n = 4). (C) Effect of p38 and ERK inhibitors on neutrophil IL-8 production in the presence of KU-55933 in response to LPS stimulation. IL-8 concentration in supernatants was measured after 18 hours of stimulation (n = 5) (D). Quantitative real-time PCR analysis of relative IL-8 and MIP-1α transcripts in cDNA from neutrophils stimulated with LPS with and without KU-55933. mRNA was isolated from LPS-stimulated neutrophils at the indicated time points, converted to cDNA, and analyzed by qPCR in triplicate (n = 3). Data are expressed as the relative amount of cytokine transcript divided by the relative amount of housekeeping gene β2-microglobulin (n = 3). Relative transcript amounts were calculated using a standard curve made from serial dilutions of a pooled sample. Data were analyzed using StepOnePlus software. (D) Intracellular IL-8 staining of LPS-stimulated neutrophils in the presence of KU-55933. Neutrophils were incubated with Brefeldin A to block secretion and stimulated with LPS with or without ATM inhibitor. At the indicated time points, cells were fixed and stained with an anti-IL8 antibody and analyzed by flow cytometry. At least 10 000 cells were analyzed per sample. Gray fill represents unstimulated neutrophils, the solid line represents LPS-stimulated dimethyl sulfoxide control, and the dashed line represents LPS-stimulated with 10 µM KU-55933. (B-D) Data are presented as the mean ± SEM of compiled experiments. Asterisks indicate significance: *P < .05, **P < .01, ***P < .001 by paired Student t test.
Increased MAPK activation in the absence of ATM activity led to increased cytokine transcription. We observed an increase in both IL-8 and MIP-1α transcripts in the presence of KU-55933 compared with the housekeeping gene β2-microglobulin by qPCR (Figure 3D). Correspondingly, we observed an increase in IL-8 protein in neutrophils stimulated in the presence of KU-55933, as shown by intracellular antibody staining and analysis by flow cytometry (Figure 3E).
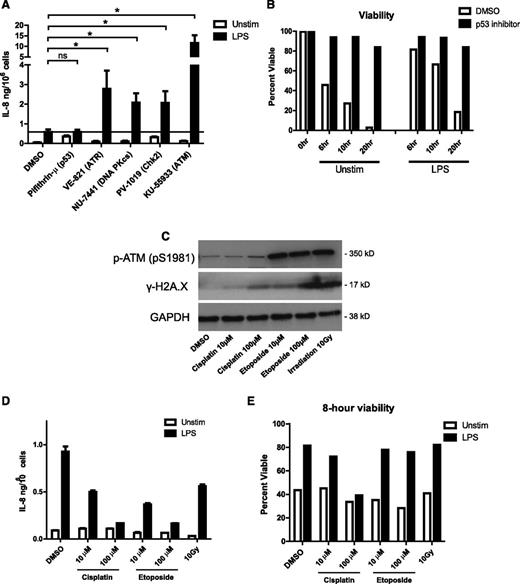
We next used a panel of small-molecule inhibitors targeting specific kinases involved in the DNA damage response to determine whether other members of this pathway influence cytokine production. Inhibition of ATR, DNA-PK, and Chk2 increased IL-8 production in response to LPS, similar to ATM inhibition and consistent with their partially redundant roles in the DNA damage response (Figure 4A). In line with this, ATM and ATR inhibition synergistically increase cytokine production above the level of either inhibitor alone (supplemental Figure 5). Interestingly, inhibiting p53 drastically prolonged neutrophil life span (Figure 4B) but did not affect IL-8 production (Figure 4A). Thus, increased cytokine production in the absence of DNA damage signaling is not strictly linked to prolonged lifespan.
Activation of the DNA damage response downregulates neutrophil cytokine production. (A) Neutrophil IL-8 production in response to LPS stimulation in the presence of inhibitors of DNA damage-response proteins. Neutrophils were incubated with the indicated inhibitors (inhibitor targets in parentheses) and stimulated 18 hours with LPS in triplicate. IL-8 concentrations in supernatants were measured by ELISA. Data are plotted as the mean ± SEM of compiled experiments (n = 5). The black line represents the amount of IL-8 produced by LPS-stimulated control cells. (B) Neutrophil viability time course in the presence of p53 inhibitor pifithrin-µ (1 µM) measured by PE-annexin V/PI staining, as before. (C) Western blot analysis of phosphorylation of ATM and histone H2A.X in neutrophil lysates made after 1 hour of treatment with cisplatin, etoposide, and γ-irradiation. (D) Neutrophil IL-8 production in response to 18-hour LPS stimulation after 1 hour exposure to the indicated DNA damage–inducing agents. Data are plotted as the mean ± standard deviation. Results are representative of 3 experiments. (E) Neutrophil viability after 1 hour of incubation with indicated DNA damage–inducing treatment followed by 8 hours of stimulation with LPS or left unstimulated. Viability was measured as described by PE-annexin V/PI staining. Asterisks indicate significance: *P < .05, **P < .01, ***P< . 001 by paired Student t test.
Activation of the DNA damage response downregulates neutrophil cytokine production. (A) Neutrophil IL-8 production in response to LPS stimulation in the presence of inhibitors of DNA damage-response proteins. Neutrophils were incubated with the indicated inhibitors (inhibitor targets in parentheses) and stimulated 18 hours with LPS in triplicate. IL-8 concentrations in supernatants were measured by ELISA. Data are plotted as the mean ± SEM of compiled experiments (n = 5). The black line represents the amount of IL-8 produced by LPS-stimulated control cells. (B) Neutrophil viability time course in the presence of p53 inhibitor pifithrin-µ (1 µM) measured by PE-annexin V/PI staining, as before. (C) Western blot analysis of phosphorylation of ATM and histone H2A.X in neutrophil lysates made after 1 hour of treatment with cisplatin, etoposide, and γ-irradiation. (D) Neutrophil IL-8 production in response to 18-hour LPS stimulation after 1 hour exposure to the indicated DNA damage–inducing agents. Data are plotted as the mean ± standard deviation. Results are representative of 3 experiments. (E) Neutrophil viability after 1 hour of incubation with indicated DNA damage–inducing treatment followed by 8 hours of stimulation with LPS or left unstimulated. Viability was measured as described by PE-annexin V/PI staining. Asterisks indicate significance: *P < .05, **P < .01, ***P< . 001 by paired Student t test.
These data suggest that DNA damage signaling downregulates neutrophil cytokine production. To directly test this hypothesis, we preincubated neutrophils for 1 hour with cisplatin or etoposide, 2 chemical inducers of DNA damage, or exposed them to 10 Gy of γ-irradiation before stimulating them with LPS. These treatments induced DNA damage and activated ATM, as shown by Western blot analysis of histone γ-H2A.X and phospho-ATM (Figure 4C), and significantly reduced the production of IL-8 (Figure 4D). Interestingly, treatment with etoposide or irradiation strongly activated ATM and reduced cytokine production without large effects on cell viability after 8 hours (Figure 4E). Together, these results show that activation of the DNA damage response suppresses cytokine production and contributes to neutrophil apoptosis.
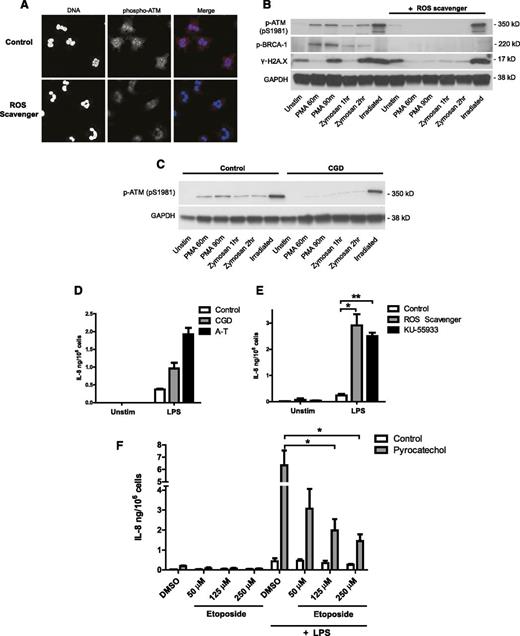
Because ROS can cause oxidative damage to DNA, we hypothesized that the oxidative burst mounted upon stimulation may activate the DNA damage response in neutrophils. We tested this by using PMA and opsonized zymosan, 2 potent inducers of the oxidative burst (supplemental Figure 6). In response to PMA, we observed an accumulation of phospho-ATM staining in the nuclei of stimulated neutrophils (supplemental Figure 7). We tested the ROS dependence of this activation using pyrocatechol (Figure 5A), a cell-permeable ROS scavenger capable of neutralizing the oxidative burst as shown in supplemental Figure 8. In the presence of pyrocatechol, activated neutrophils showed less phosphorylation of ATM compared with controls (Figure 5A). We confirmed the activation of DNA damage signaling by Western blot analysis in neutrophils stimulated with PMA and opsonized zymosan (Figure 5B). Stimulation caused a strong induction of phospho-ATM, phospho-BRCA-1 (a substrate of ATM), and γ-H2A.X in control cells, but not in the presence of pyrocatechol. Phosphorylation of ATM and H2A.X in response to irradiation-induced DNA damage, however, was not affected by ROS scavenging.
Neutrophil oxidative burst activates the DNA damage response and regulates cytokine production. (A-D) NADPH oxidase-derived ROS activates the DNA damage response in stimulated neutrophils. (A) Phospho-ATM (red) and DNA (blue) staining of healthy neutrophils activated with PMA in the presence of 30 μM ROS scavenger pyrocatechol. neutrophils were stimulated for 90 minutes with PMA to induce ROS production, fixed, permeabilized, stained with anti-pATM (pS1981) antibody, and visualized by confocal microscopy. (B-D) Western blot analysis of DNA damage-response activation in lysates from neutrophils stimulated with PMA, opsonized zymosan, or 10 Gy irradiation in the absence or presence of pyrocatechol (B), or isolated from control and CGD patient (C). (D-E) IL-8 production from neutrophils deficient in ATM activity and ROS production. (D) IL-8 production from neutrophils isolated concurrently from a CGD patient, an AT patient, and a healthy control after 18 hour of LPS stimulation (single experiment). Data are plotted as the mean ± SEM of triplicate stimulations. (E) IL-8 production from healthy control neutrophils incubated with ROS scavenger pyrocatechol or ATM inhibitor KU-55933, and stimulated for 18 hours with LPS (n = 3). IL-8 concentration in supernatants was measured by ELISA. (F) IL-8 production from neutrophils incubated with pyrocatechol, pretreated for 1 hour with indicated concentrations of etoposide, and stimulated for 18 hours with LPS (n = 3). (E-F) Data are presented as the mean ± SEM of compiled experiments. Asterisks indicate significance: *P < .05, **P < .01, ***P < .001 by paired Student t test.
Neutrophil oxidative burst activates the DNA damage response and regulates cytokine production. (A-D) NADPH oxidase-derived ROS activates the DNA damage response in stimulated neutrophils. (A) Phospho-ATM (red) and DNA (blue) staining of healthy neutrophils activated with PMA in the presence of 30 μM ROS scavenger pyrocatechol. neutrophils were stimulated for 90 minutes with PMA to induce ROS production, fixed, permeabilized, stained with anti-pATM (pS1981) antibody, and visualized by confocal microscopy. (B-D) Western blot analysis of DNA damage-response activation in lysates from neutrophils stimulated with PMA, opsonized zymosan, or 10 Gy irradiation in the absence or presence of pyrocatechol (B), or isolated from control and CGD patient (C). (D-E) IL-8 production from neutrophils deficient in ATM activity and ROS production. (D) IL-8 production from neutrophils isolated concurrently from a CGD patient, an AT patient, and a healthy control after 18 hour of LPS stimulation (single experiment). Data are plotted as the mean ± SEM of triplicate stimulations. (E) IL-8 production from healthy control neutrophils incubated with ROS scavenger pyrocatechol or ATM inhibitor KU-55933, and stimulated for 18 hours with LPS (n = 3). IL-8 concentration in supernatants was measured by ELISA. (F) IL-8 production from neutrophils incubated with pyrocatechol, pretreated for 1 hour with indicated concentrations of etoposide, and stimulated for 18 hours with LPS (n = 3). (E-F) Data are presented as the mean ± SEM of compiled experiments. Asterisks indicate significance: *P < .05, **P < .01, ***P < .001 by paired Student t test.
To test whether the oxidative burst is the endogenous source of ROS activating the DNA damage response, we similarly evaluated ATM activation in neutrophils from patients with chronic granulomatous disease (CGD). CGD is a genetic disorder in which neutrophils fail to mount an oxidative burst because of deficiencies in NADPH oxidase (supplemental Figure 9). Interestingly, we could show that ATM is effectively activated in neutrophils isolated from CGD patients in response to irradiation, but not in response to ROS-inducing stimuli (Figure 5C). This confirms that NADPH oxidase–derived ROS are required for activating the DNA damage response in stimulated neutrophils.
Our results predict that CGD neutrophils, similar to AT neutrophils, would produce more cytokines as a result of impaired activation of ATM and the DNA damage response. We confirmed this by comparing cytokine production from patients with the 2 deficiencies: neutrophils isolated concurrently from both a CGD and an AT patient produced more IL-8 than cells from a healthy control (Figure 5D). We obtained similar results when incubating neutrophils with ROS scavenger pyrocatechol or ATM inhibitor KU-55933 (Figure 5E). This is consistent with published reports on the hyperactivation of CGD phagocytes.32-34 Furthermore, we could rescue the IL-8 overproduction phenotype of ROS-scavenged cells by exogenously inducing DNA damage (Figure 5F). Etoposide treatment potently decreased IL-8 production from pyrocatechol-treated neutrophils back to levels similar to control cells.
Discussion
Our study is, to the best of our knowledge, the first to examine primary innate immune cells from AT patients. We show that ROS-mediated activation of DNA damage signaling in neutrophils suppresses the production of proinflammatory cytokines and activates apoptosis. Accordingly, we report that deficiencies in the DNA damage response, similar to deficiencies in the oxidative burst, result in the overproduction of cytokines and delayed apoptosis. We demonstrate a key role for ATM and show that cytokine production and apoptosis are dysregulated in neutrophils from patients with AT. These results further our understanding of the ROS-mediated regulation of inflammation and indicate an interesting link between 2 diverse diseases, AT and CGD. Moreover, our results suggest that innate immune dysfunction may drive inflammation in AT.
The specific mechanisms by which ATM and the DNA damage response contribute to the regulation of cytokine production are not completely clear. The effect is at least partially mediated by modulating well-described MAPK signaling pathways. Our data demonstrate an important role for p38 and suggest one for ERK as well. It has previously been reported that ATM and other members of the DNA damage response can affect the activation of MAP kinases and NF-κB,35,36 which are important regulators of cell survival and inflammation.37
Consistent with our findings, several lines of evidence have recently emerged that point to ATM having a major role in suppressing inflammation. In human dendritic cells ATM was shown to be a negative regulator of IL-23 production.38 Furthermore, pharmacologic induction of the DNA damage response reduces levels of proinflammatory cytokines and increases survival in septic mice.39 This protective effect was shown to be partly mediated by ATM activation, although the researchers conclude that this is a lung-specific mechanism not mediated by blood cells. Similarly, Härtlova et al40 reported that ATM deficiency in macrophages increases production of type I interferon. The authors, however, do not suggest a direct role for ATM in interferon regulation. Rather, they propose that unrepaired DNA lesions in AT cells activate Stimulator of IFN Genes (STING) and prime the type I interferon system. This mechanism and the ROS-mediated one proposed by our study are not mutually exclusive. It would be interesting to examine whether other cytokines, including IL-8, are also upregulated in AT macrophages.
Our data indicate that ATM is not the sole member of the DNA damage response that regulates neutrophil function. Inhibition of DNA damage sensors ATR and DNA-PK, as well as Chk2, a substrate of ATM, also increased cytokine production. These sensors are often described as each recognizing a specific type of damage to DNA and executing parallel but discrete responses through different downstream mediators. In reality, there is much overlap and crosstalk between these pathways and substrates, meaning they may be partially redundant.41 Indeed, we observe that simultaneous inhibition of ATM and ATR synergistically increases cytokine production more than either inhibitor alone (supplemental Figure 5). Chk2 has been shown to affect MAP kinase activation42,43 and can also be activated by ATR, though less efficiently than by ATM.44 Thus, these kinases may converge on Chk2 to regulate cytokine production.
Neutrophil cytokine production and lifespan are closely linked and may affect each other. Secretion of IL-8 can increase lifespan by autocrine signaling45 and is one possible mechanism causing increased viability in AT cells. We can, however, conclude that the regulation of cytokine production by the DNA damage response can be uncoupled from its regulation of apoptosis. Inhibiting p53 dramatically prolonged neutrophil lifespan, but did not increase IL-8 production. Furthermore, normalizing cytokine production to viable cells at an earlier time point clarifies that when ATM is inhibited, the production of IL-8 increases in magnitude and is not only a result of an increased lifespan (supplemental Figure 10). Similarly, inducing DNA damage by treatment with etoposide, or irradiation, reduced cytokine production, but had no effect on apoptosis in the time span under investigation.
One of the most prominent neutrophil responses to activating stimuli, in addition to cytokine production, is the oxidative burst. Production of superoxide and its derivatives hydrogen peroxide and hypochlorous acid is thought to contribute to microbial killing and also serve important signaling functions.23 ROS are known to induce DNA damage and to activate ATM directly by oxidation21 and were thus prime candidates for triggering ATM activity in activated neutrophils. By comparing neutrophil responses in AT and CGD patients, we identified the oxidative burst as an activator of ATM important for regulating cytokine production. A side-by-side comparison of IL-8 production in control, CGD, and AT neutrophils reveals that ROS and ATM deficiencies phenocopy each other in this respect. Exogenous induction of DNA damage can rescue the cytokine overproduction phenotype of ROS-scavenged neutrophils and further supports this model. It is not yet clear whether the activation of ATM by ROS is directly mediated by oxidation of ATM, or via oxidative damage to the DNA. Phosphorylation of H2A.X in response to LPS when ATM is inhibited indicates that this activation is via DNA damage and may be increased in ATM deficiency as a result of inefficient repair.
Our results thus suggest the following model for the initiation and resolution of inflammation by myeloid cells: (1) neutrophil activation leads to activation of MAP kinases and initiation of oxidative burst and cytokine production; (2) NADPH oxidase–derived ROS, in addition to contributing to microbial killing, also activate ATM; and (3) ATM signaling participates in initiating the resolution phase by limiting cytokine production and promoting antiinflammatory neutrophil cell death. Thus, augmented cytokine production coupled with decreased apoptosis rates could result in a synergistic increase in the magnitude and lifespan of neutrophil inflammatory responses in AT patients.
Furthermore, our model suggests a potential mechanistic link between the pathologies observed in 2 completely different diseases: AT and CGD (supplemental Figure 11). ROS are ubiquitous and promiscuous molecules, playing a role in signaling and regulation of many pathways. In neutrophils, the oxidative burst is known to regulate antimicrobial responses, apoptosis, autophagy, hypoxia and oxidative stress responses, chemotaxis, and NETosis.23 CGD is the result of dysregulation of all of these responses, which causes immunodeficiency and hyperinflammation. Similarly, AT is the result of dysfunctions in many pathways involving ATM. We propose that the ROS-mediated activation of ATM is an important response that is dysregulated in AT that affects cytokine production, a dysregulation that is shared by patients with CGD. Though these diseases are very different, they may have an inflammatory common denominator.
Chronic inflammation, granuloma formation, and elevated risk of autoimmune disorders in CGD are attributed to increased cytokine production and delayed apoptosis of neutrophils and macrophages.34 We observe similar dysregulation of neutrophil functions in AT patients and believe this may also drive chronic inflammation and disease in AT. In agreement with this, several authors have reported instances of nonresolving, sterile skin granulomas in AT patients. Furthermore, both AT and CGD patients have an elevated risk of developing autoimmune diseases and autoantibodies.46-49 Prolonged inflammatory responses could drive and exacerbate the inflammatory symptoms of AT and even contribute to neurodegeneration and high rates of cancer. Several reports have suggested excessive inflammatory responses in a mouse model of AT, resulting in increased susceptibility to colitis,50 as well as inflammatory tissue damage in the lung.51
There is currently no treatment available for AT other than palliative care. Interestingly, Chessa et al52 found that treatment with the antiinflammatory steroid betamethasone improved neurological symptoms for AT patients, though it remains to be seen whether this effect was mediated by the immunosuppressive properties of the drug. Our work further strengthens the argument that targeting inflammation may be a promising clinical intervention for some of the symptoms of this devastating disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the AT and CGD patients for their participation in this study; Martin Digweed for valuable advice and discussion about the project; and Bärbel Raupach, Gabriel Sollberger, Elaine Kenny, and Lorenz Knackstedt for their constructive comments on the manuscript.
This work was supported by the Max Planck Society, and an EMBO long-term fellowship (B.A.).
Authorship
Contribution: C.J.H. and B.A. designed and performed the experiments and analyzed the data; C.J.H. made the figures and wrote the manuscript; P.V.S.-P., H.v.B., A.M.K., B.T.C.-C., A.C.-N., J. Reichenbach and J. Roesler arranged for blood donation by AT and CGD patients, provided facilities for experiments, and contributed helpful comments to the manuscript; and A.Z. and B.A. directed the study and supervised the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Borko Amulic, Max Planck Institute for Infection Biology, Chariteplatz 1, 10117 Berlin, Germany; e-mail: amulic@mpiib-berlin.mpg.de.
References
Author notes
A.Z. and B.A. contributed equally to this study.