Key Points
As little as 5% of kindlin-3 is sufficient to maintain basal platelet and leukocyte functions.
Platelets and neutrophils contain stoichiometric quantities of kindlin-3 and talin-1.
Abstract
Hematopoietic cells depend on integrin-mediated adhesion and signaling, which is induced by kindlin-3 and talin-1. To determine whether platelet and polymorphonuclear neutrophil (PMN) functions require specific thresholds of kindlin-3, we generated mouse strains expressing 50%, 10%, or 5% of normal kindlin-3 levels. We report that in contrast to kindlin-3–null mice, which die perinatally of severe bleeding and leukocyte adhesion deficiency, mice expressing as little as 5% of kindlin-3 were viable and protected from spontaneous bleeding and infections. However, platelet adhesion and aggregation were reduced in vitro and bleeding times extended. Similarly, leukocyte adhesion, extravasation, and bacterial clearance were diminished. Quantification of protein copy numbers revealed stoichiometric quantities of kindlin-3 and talin-1 in platelets and neutrophils, indicating that reduction of kindlin-3 in our mouse strains progressively impairs the cooperation with talin-1. Our findings show that very low levels of kindlin-3 enable basal platelet and neutrophil functions, whereas in stress situations such as injury and infection, platelets and neutrophils require a maximum of functional integrins that is achieved with high and stoichiometric quantities of kindlin-3 and talin-1.
Introduction
Integrins are cell adhesion receptors that consist of α and β subunits. They anchor cells to the extracellular matrix and assemble large signaling hubs, with which they regulate essential cellular processes including cell adhesion, migration, proliferation, survival, and differentiation. A hallmark of integrins is their ability to reversibly switch between an active and an inactive conformation. In their inactive conformation, they have low affinity for ligands, whereas upon activation (inside-out signaling), the affinity increases, leading to ligand binding, integrin clustering, and, finally, signaling (outside-in signaling). Two principal protein families control integrin activity, talins (talin-1 and -2) and kindlins (kindlin-1, -2, -3). They both bind directly to β-integrin tails, cooperate during integrin inside-out and outside-in signaling, and stabilize the integrin-ligand interaction by inducing integrin clustering.1-5
A fast allosteric change and stable integrin-ligand interaction is particularly important for blood cells such as platelets and leukocytes, whose surface integrins continuously encounter ligands in plasma or on the vascular endothelium, respectively. Therefore, it is of fundamental importance for these cells to rapidly activate their integrins on demand—for example, during bleeding when platelet aggregation is needed to seal vascular injuries, or during infection when leukocytes need to extravasate and kill microbial invaders. Consequently, loss of talin-1 and kindlin-3, which are expressed in all hematopoietic cells, leads to severe bleeding and immunodeficiency disorders.6-11 Despite this central role of talin-1 and kindlin-3 in controlling integrin activity, it is unclear whether they are expressed and bind integrin tails in stoichiometric quantities, whether they are present in excess over integrins in hematopoietic cells and whether a concentration threshold is required for them to enable integrin-mediated cellular processes to proceed normally.
In the present study, we engineered several mouse strains expressing different kindlin-3 protein levels. This allowed testing whether different talin-1/kindlin-3 ratios influence integrin-mediated functions. We found that 5% of normal kindlin-3 levels in hematopoietic cells are sufficient for embryonic and postnatal development. However, upon exposure to stress, the low levels of kindlin-3 impair adhesive functions of platelets and neutrophils. We also found that kindlin-3 and talin-1 are expressed in stoichiometric quantities in both cell types. By contrast, there are twice as many β2-integrin molecules and only half the number of β3 integrins as talin-1/kindlin-3 in neutrophils and platelets, respectively. The implications of these findings are discussed.
Material and methods
Animals
Kindlin-3–deficient,7 β3-integrin–deficient,12 talin-1 floxed,13 and Mx-1 transgenic14 mice were described previously. Cre expression in the hematopoietic system was induced by intraperitoneal injection of 250 mg poly-I/C (GE Healthcare, Munich, Germany).
To generate conditional kindlin-3 mouse mutants (kindlin-3 fl/fl), a targeting vector was cloned, in which exons 3 to 6 of the kindlin-3 gene were flanked by loxP sites. This vector was electroporated into 129 ES cells. A Neo-cassette flanked by frt sites allowed G418 selection. Several homologous recombinant clones were injected into C57BL/6 blastocysts and resulted in germline chimeras. The presence of the Neo-cassette within the kindlin-3 genomic locus resulted in strongly reduced kindlin-3 expression, giving rise to a hypomorphic kindlin-3 allele (denominated n). Mating with mice carrying a wild type (K3+/+), hypomorphic (K3+/n; K3n/n; K3n/−), or kindlin-3–null (K3−/−) alleles resulted in offspring with different levels of kindlin-3. In parallel, the Neo-cassette was removed from the kindlin-3 genomic locus by mating kindlin-3 hypomorphic mice with a mouse strain expressing a deleter flippase, resulting in conditional kindlin-3 mice. Finally, conditional kindlin-3 mice were crossed with a deleter Cre mouse strain15 to obtain K3−/− mice. Mouse experiments were performed with the approval of the District Government of Bavaria.
Adhesion assays
For PMN adhesion to ICAM-1, 96-well plates were coated with 4 µg/mL recombinant human ICAM-1 (R&D Systems) in coating buffer (150 mM NaCl, 20 mM Tris-HCl, and 2 mM MgCl2, pH 9) ON at 4°C and blocked with 3% bovine serum antigen in phosphate-buffered saline for 1 hour at room temperature. PMNs sorted from bone marrow were either left untreated or treated either with 33 ng/mL phorbol-12-myristate-13-acetate (PMA) (Calbiochem, Merck-Millipore) or 1 mM MnCl2. The adhesion assay was performed with 50 000 cells per well for 30 minutes. Adherent cells were fixed with 4% paraformaldehyde after washing. The number of adherent cells was determined by acquisition of 3 phase-contrast pictures from each well.
Phagocytosis assay
Phagocytosis was measured using the pHrodoTM Red Escherichia coli BioParticle Phagocytosis Kit for Flow cytometry (Invitrogen, Darmstadt, Germany) according to the manufacturer’s instructions. Blood was collected from the retro-orbital sinus and each blood sample was measured in triplicate. Granulocytes were identified by their position in a forward-vs-side scatter plot.
Helicobacter pylori infection model
Animals between 6 and 12 weeks of age were infected orogastrically 3 times on 3 consecutive days with a dose of 109H pylori strain PMSS1. Wild-type littermate controls were inoculated with identical volumes of sterile Brucella broth (BB) alone. All experiments and procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Regierung von Oberbayern. Animals were sacrificed and analyzed 3 months after the infection.
To investigate the bacterial load, stomachs were opened along the greater curvature and washed in phosphate-buffered saline. The whole stomach was divided into 2 equal halves. One half was homogenized (glass homogenizer from Ochs, Bovenden, Germany) in BB, and the other half was fixed in formalin and used for histopathology. Appropriate dilutions of homogenates were spread on selective serum plates (GC agar Difco, BD Biosciences) supplemented with horse serum (80 mL/L), IsoVitaleX (10 mL/L; both from BD Biosciences), nalidixic acid (5 g/L), bacitracin (50 g/L), and the H pylori selective medium DENT (vancomycin [10 mg/L], trimethoprim [5 mg/L], cefsulodin [5 mg/L], amphotericin B [5 mg/L]; all from Oxoid, Thermo Scientific, Wesel, Germany). Plates were incubated under microaerophilic conditions (85% N2, 10% CO2, 5% O2) at 37°C for up to 5 days. The number of colony-forming units was calculated per gram of gastric tissue.
Histomorphologic analysis was done on hematoxylin and eosin–stained longitudinal sections of the whole stomach. The activity of gastritis and the intensity of inflammation (scale 0-3) were analyzed according to Garhart et al.16 Furthermore, the immigrated granulocytes were counted in a high-power field (HPF) in the area of maximum inflammation.
Absolute quantification of proteins in platelets and neutrophils
We produced 2 protein standards spanning different regions of talin-1, β3 and β2 integrins, and 3 standards for kindlin-3. The protein standards were designed with an approximate length of 150 amino acids and with as many unique tryptic cleavage sites as possible. They covered in kindlin-3 the amino acids 114-276, 304-477, and 522-605; in talin-1 1752-1948 and 2021-2188; in β3 integrin 34-162 and 589-714; and in β2 integrin 176-325 and 334-480. Subsequently, they were fused to the albumin-binding protein (ABP), whose cDNA was inserted into the expression vector pET28a(+) and then fused with the gene sequences of interest using HindIII and XhoI. In a next step, the proteins fused to ABP were expressed in an auxotrophic E coli strain and labeled with “heavy” isotopes (13C615N2-lysine and 13C615N4-arginine). The labeled proteins were purified via His6-Tag using spin columns from QIAGEN (Hilden, Germany).
The proteins of interest were quantified as previously described.17 In brief, unlabeled ABP was quantified beforehand with amino acid analysis. These measurements were used to quantify the “heavy” protein standards via SILAC ratios. The different protein standards with known concentrations were then mixed. The platelets or neutrophils were lysed and the “protein standard mix” was spiked in at the approximately endogenous level. The mixture was further processed using the filter-aided sample preparation method,18 in which proteins were captured on a 30-kDa filter and sodium dodecyl sulfate was removed with a urea-containing buffer. Proteins were alkylated with iodoacetamide and trypsinized. The peptides were measured by mass spectrometry (MS), and peptide ratios between endogenous and labeled peptides were extracted.
For the platelet proteome, filter-aided sample preparation peptides were further fractionated with strong anion exchange chromatography. Six fractions of pH 11, 8, 6, 5, 4, and 3 were obtained.
All samples were measured using the LTQ-Orbitrap Elite or Q Exactive proteomic pipeline.19 Raw mass spectrometric data were analyzed using the MaxQuant software.20 A detailed description of the MS analysis, as well as the data analysis, can be found in the supplemental material, found on the Blood Web site.
For calculation of the absolute copy number per cell, we used the peptide ratios and converted the ratios to pmol. We took the median of all peptides amounts (derived from different protein standards) to calculate the copy number. We required at least 3 peptides for the quantification of each protein.
Statistical analysis
All data are shown as mean ± standard deviation (SD) or mean ± standard error of the mean (SEM) as indicated in the figure legends. To test significance level, an unpaired Student t test was performed. The data of the H pylori inflammation model were analyzed with the Mann-Whitney U test for unpaired groups. Significant differences are indicated (*P < .05, **P < .01, ***P < .001).
Results
Generation of conditional and hypomorphic kindlin-3 mouse mutants
Kindlin-3–deficient mice die shortly after birth of severe anemia caused by bleeding and erythrocyte defects.1,8 To enable the disruption of the kindlin-3 gene at later stages or in specific hematopoietic cells, we generated a conditional kindlin-3 mouse by flanking exon 3-6 with loxP sites (supplemental Figure 1A-C).21 An intercross of these kindlin-3 floxed (K3fl/fl) mice with a deleter Cre strain produced offspring lacking exon 3-6 and the frt-flanked neomycin cassette, which, as expected, had the same defects observed in constitutive kindlin-3–null mice1,22 (supplemental Figure 1D-E). We noticed, however, that the presence of the neomycin cassette in intron 6 of the floxed kindlin-3 gene (supplemental Figure 1A; K3+/n mice) reduced kindlin-3 protein levels in thymus and spleen (Figure 1A-B). Intercrossing these mice to homozygosity (K3n/n) or with mice carrying a kindlin-3–null allele (K3n/−) further reduced kindlin-3 protein levels in the spleen and thymus to ∼10% and 5%, respectively (Figure 1A-B). Sequence analysis of kindlin-3 mRNA revealed aberrant splicing into the neomycin cassette as a major cause for reduced protein expression (data not shown). Thus, the introduction of a neomycin cassette into intron 6 of the kindlin-3 gene produced a hypomorphic kindlin-3 allele, and by intercrossing these mice with wild-type or kindlin-3–null mice, we could differently reduce kindlin-3 protein levels.
Kindlin-3 protein levels in spleen and thymus. (A) Western blot analyses of kindlin-3 in spleen and thymus lysates from K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− mice. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading control. (B) Densitometric quantification of (A). Values are given as mean ± SD.
Kindlin-3 protein levels in spleen and thymus. (A) Western blot analyses of kindlin-3 in spleen and thymus lysates from K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− mice. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading control. (B) Densitometric quantification of (A). Values are given as mean ± SD.
Surprisingly, the different hypomorphic mouse strains including the K3n/− mice, with 5% expression of kindlin-3 in the thymus and spleen, were born with the normal Mendelian ratio, developed and aged normally, were fertile, and showed normal cellularity in the spleen and thymus (supplemental Figure 2A-C). Furthermore, their erythroid counts were normal, indicating that they did not suffer from small bleeds or erythrocyte defects (supplemental Figure 3). However, the total number of white blood cells (WBCs), in particular PMNs and lymphocytes, was significantly increased in K3n/n and K3n/− mice, suggesting that low kindlin-3 levels compromise leukocyte extravasation (Table 1). In contrast to a previous report,23 we could not detect kindlin-3 protein in vascular endothelial cells. Therefore, we refrained from investigating kindlin-3 function(s) in endothelial cells (supplemental Figure 4).
Blood counts of K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− chimeras
| . | +/+( n = 9) . | +/n (n = 8) . | n/n (n = 9) . | n/− (n = 9) . | −/−chim (n = 4) . |
|---|---|---|---|---|---|
| (103/µL) WBC | 5.70 ±2.45 | 6.38 ±2.05 | 13.30 ±2.58 | 9.76 ±4.01 | 18.23 ±2.97 |
| (103/µL) NE | 1.01 ±0.49 | 1.21 ±0.46 | 4.15 ±1.48 | 2.53 ±2.38 | 13.97 ±2.99 |
| (103/µL) LY | 4.32 ±2.09 | 4.72 ±1.49 | 8.37± 2.27 | 6.62 ±3.68 | 3.33 ±0.55 |
| (106/µL) RBC | 9.44 ±1.27 | 9.19± 0.82 | 8.35 ±1.21 | 8.35± 0.95 | 2.44 ±0.47 |
| (g/dL) Hb | 13.93 ±2.29 | 13.56 ±1.19 | 12.56 ±2.12 | 12.71 ±1.53 | 5.78 ±0.77 |
| (106/µL) PLT | 603± 138 | 452 ±62 | 593 ±112 | 695 ±216 | 648 ±167 |
| . | +/+( n = 9) . | +/n (n = 8) . | n/n (n = 9) . | n/− (n = 9) . | −/−chim (n = 4) . |
|---|---|---|---|---|---|
| (103/µL) WBC | 5.70 ±2.45 | 6.38 ±2.05 | 13.30 ±2.58 | 9.76 ±4.01 | 18.23 ±2.97 |
| (103/µL) NE | 1.01 ±0.49 | 1.21 ±0.46 | 4.15 ±1.48 | 2.53 ±2.38 | 13.97 ±2.99 |
| (103/µL) LY | 4.32 ±2.09 | 4.72 ±1.49 | 8.37± 2.27 | 6.62 ±3.68 | 3.33 ±0.55 |
| (106/µL) RBC | 9.44 ±1.27 | 9.19± 0.82 | 8.35 ±1.21 | 8.35± 0.95 | 2.44 ±0.47 |
| (g/dL) Hb | 13.93 ±2.29 | 13.56 ±1.19 | 12.56 ±2.12 | 12.71 ±1.53 | 5.78 ±0.77 |
| (106/µL) PLT | 603± 138 | 452 ±62 | 593 ±112 | 695 ±216 | 648 ±167 |
Hb, hemoglobin; LY, lymphocyte; NE, neutrophil; PLT, platelet; RBC, red blood cell; WBC, white blood cell.
Values are shown as mean ± SD.
Kindlin-3 hypomorphic mice show a bleeding tendency
Kindlin-3 deficiency causes fatal bleeds in mice and humans.7,9-11 Although platelets from K3n/n and K3n/− mice expressed ∼10% and 5% of kindlin-3 levels compared with wild-type controls (Figure 2A), they did not suffer from spontaneous hemorrhages during development or postnatal life (Figure 2B, and data not shown). However, bleeding assays revealed that wild-type and K3+/n mice stopped bleeding within 5 minutes, whereas K3n/n mice suffered from a severe bleeding tendency that was comparable with kindlin-3–null chimeric mice (Figure 2C). Thrombin treatment (0.1 U/mL) of wild-type and K3+/n platelets robustly induced fibrinogen binding by platelet αIIbβ3 integrin, whereas fibrinogen binding was reduced to <30% in K3n/n and 20% in K3n/− platelets and was almost entirely lost in K3−/− platelets (Figure 2D). Using a higher thrombin concentration (1 U/mL), increased fibrinogen binding slightly to 35% in K3n/n and 25% in K3n/− compared with wild-type platelets (Figure 2E). Thrombin-induced activation (0.1 U/mL) of αIIbβ3 integrins measured by flow cytometry using the JON/A-PE antibody decreased concomitantly with lowering kindlin-3 expression levels in platelets (Figure 2F). Of note, a tenfold increase of thrombin (1 U/mL) increased the number of active αIIbβ3 integrins on kindlin-3 hypomorphic and deficient platelets relative to wild-type platelets (Figure 2G). Furthermore, the activation of β1 integrins measured with the β1-integrin activation epitope reporting antibody 9EG7 also increased in a kindlin-3 dose-dependent manner (Figure 2H). Interestingly, low levels of JON/A-PE–binding and hence active αIIbβ3 integrins were measureable even in the absence of kindlin-3 and especially after high-dose thrombin treatment (Figure 2F-H). This finding prompted us to test whether talin-1–deficient platelets also display active αIIbβ3 integrins on their surface after thrombin treatment. Indeed, when we measured JON/A-PE binding on talin-1–deficient platelets, we observed binding, although less than on kindlin-3–null platelets (Figure 2I-J). Importantly, despite the presence of JON/A-PE–binding integrins, neither kindlin-3– nor talin-1–deficient platelets were able to form stable platelet aggregates (Figure 2K). Thus, the low levels of JON/A-PE–binding and hence active αIIbβ3 integrins on talin-1– and kindlin-3–deficient platelets suggest that either kindlin-3 or talin-1 alone is sufficient to shift a small amount of integrins into an active conformation but is insufficient to maintain/stabilize the active conformation.
Function of platelet integrins is kindlin-3 dose-dependent. (A) Densitometric quantification of kindlin-3 levels in K3+/+, K3+/n, K3n/n, and K3n/− platelets (N = 5, 5, 4, 4). GAPDH served as loading control. (B) K3+/+, K3+/n, K3n/n, K3n/− E14.5 embryos are free of bleeding. (C) Tail bleeding times of K3+/+, K3+/n, K3n/n, and K3−/− chimeric mice. (D-E) Binding of fibrinogen to platelets after treatment with 0.1 U/mL (D) and 1 U/mL thrombin (E). Resting platelets were used as control (N = 9, 8, 8, 10, 6 [D] and N = 8, 3, 8, 8, 2 [E]). (F-G) Activation of αIIbβ3 integrin on platelets upon stimulation with 0.1 U/mL (F) and 1 U/mL (G) thrombin (N = 13, 8, 11, 16, 5 [F] and N = 15, 10, 16, 16, 4 [G]). (H) β1 integrin activation index of K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− platelets upon activation with 1 U/mL thrombin (N = 10, 7, 10, 8, 3). (I) Activation of αIIbβ3 integrin on control, kindlin-3−/−, talin-1−/−, and integrin β3−/− platelets upon stimulation with 0.1 U/mL and 1 U/mL thrombin (N = 6, 6, 6, 3). (J) Western blot analyses of talin-1 and kindlin-3 expression in lysates from wild-type (+/+), K3−/−, and talin-1−/− platelets. GAPDH served as loading control. (K) Platelet aggregation assays with +/+, K3−/−, and talin-1−/− platelets in response to 0.1 U/mL thrombin. Values are given as mean ± SD and data were statistically analyzed by Student t test. *P < .05; **P < .01; ***P < .001. n.s., not significant.
Function of platelet integrins is kindlin-3 dose-dependent. (A) Densitometric quantification of kindlin-3 levels in K3+/+, K3+/n, K3n/n, and K3n/− platelets (N = 5, 5, 4, 4). GAPDH served as loading control. (B) K3+/+, K3+/n, K3n/n, K3n/− E14.5 embryos are free of bleeding. (C) Tail bleeding times of K3+/+, K3+/n, K3n/n, and K3−/− chimeric mice. (D-E) Binding of fibrinogen to platelets after treatment with 0.1 U/mL (D) and 1 U/mL thrombin (E). Resting platelets were used as control (N = 9, 8, 8, 10, 6 [D] and N = 8, 3, 8, 8, 2 [E]). (F-G) Activation of αIIbβ3 integrin on platelets upon stimulation with 0.1 U/mL (F) and 1 U/mL (G) thrombin (N = 13, 8, 11, 16, 5 [F] and N = 15, 10, 16, 16, 4 [G]). (H) β1 integrin activation index of K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− platelets upon activation with 1 U/mL thrombin (N = 10, 7, 10, 8, 3). (I) Activation of αIIbβ3 integrin on control, kindlin-3−/−, talin-1−/−, and integrin β3−/− platelets upon stimulation with 0.1 U/mL and 1 U/mL thrombin (N = 6, 6, 6, 3). (J) Western blot analyses of talin-1 and kindlin-3 expression in lysates from wild-type (+/+), K3−/−, and talin-1−/− platelets. GAPDH served as loading control. (K) Platelet aggregation assays with +/+, K3−/−, and talin-1−/− platelets in response to 0.1 U/mL thrombin. Values are given as mean ± SD and data were statistically analyzed by Student t test. *P < .05; **P < .01; ***P < .001. n.s., not significant.
Reduced adhesion, spreading, and aggregation in kindlin-3 hypomophic platelets
We next analyzed adhesion and spreading of platelets from K3n/n and K3n/− mice on a fibrinogen-coated surface and found that both properties were severely impaired (Figure 3A). Treatment of platelets with manganese, which bypasses integrin activation,24 rescued fibrinogen binding (supplemental Figure 5) and adhesion, but not spreading (Figure 3A), indicating that the diminished kindlin-3 levels in K3n/n and K3n/− platelets impaired both integrin activation and outside-in signaling required for actin reorganization. Interestingly, adhesion-induced FAK phosphorylation on tyrosine Y397 remained unchanged in kindlin-3 hypomorphic platelets, whereas it was abolished in K3−/− platelets, suggesting that integrin-mediated FAK activation occurs even at very low kindlin-3 levels (Figure 3B). Of note, fibrinogen uptake, which depends on αIIbβ3 integrin, as well as its release, proceeded in a kindlin-3–independent manner (supplemental Figure 6).
Platelet adhesion, aggregation, and clot retraction are impaired at low kindlin-3 levels. (A) Washed platelets were stimulated with 0.01 U/mL thrombin and allowed to adhere to coated fibrinogen for 10 minutes in the presence or absence of 3 mM Mn2+. (B) Washed platelets were either left untreated or were plated on fibrinogen in the presence of 1 mM Mn2+ and 0.01 U/mL thrombin. FAK Y397 phosphorylation was densitometrically quantified from at least 3 different Western blots and normalized for GAPDH levels. Values are shown as mean ± SD. Data were statistically analyzed by Student t test. (C) Aggregates of K3+/+, K3+/n, K3n/n, and K3n/− platelets in response to 0.1 U/mL thrombin. (D) Platelet aggregation assays with K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− platelets in response to 0.1 U/mL thrombin. (E) Clot retraction of platelets from K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− mice. *P < .05; **P < .01; ***P < .001. n.s., not significant.
Platelet adhesion, aggregation, and clot retraction are impaired at low kindlin-3 levels. (A) Washed platelets were stimulated with 0.01 U/mL thrombin and allowed to adhere to coated fibrinogen for 10 minutes in the presence or absence of 3 mM Mn2+. (B) Washed platelets were either left untreated or were plated on fibrinogen in the presence of 1 mM Mn2+ and 0.01 U/mL thrombin. FAK Y397 phosphorylation was densitometrically quantified from at least 3 different Western blots and normalized for GAPDH levels. Values are shown as mean ± SD. Data were statistically analyzed by Student t test. (C) Aggregates of K3+/+, K3+/n, K3n/n, and K3n/− platelets in response to 0.1 U/mL thrombin. (D) Platelet aggregation assays with K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− platelets in response to 0.1 U/mL thrombin. (E) Clot retraction of platelets from K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− mice. *P < .05; **P < .01; ***P < .001. n.s., not significant.
Next we performed in vitro aggregation assays, in which platelets from K3n/n and K3n/− mice produced much smaller aggregates (Figure 3C) and displayed a reduced aggregation response compared with K3+/+ and K3+/n platelets (Figure 3D). Furthermore, thrombin-induced platelet clot retraction was progressively impaired with platelets from K3n/n, K3n/−, and K3−/− mice (Figure 3E).
Altogether, these data indicate that lowering kindlin-3 in platelets to 5% to 10% of wild-type levels allows them to activate a limited number of αIIbβ3 and β1 integrins that is sufficient to fulfill basal functions but is insufficient to cope with stress conditions.
K3n/n and K3n/− mice suffer from impaired leukocyte adhesion, extravasation, and bacterial clearance
Absence of kindlin-3 severely impairs β2-integrin functions on leukocytes, resulting in leukocyte adhesion deficiency in mouse and human.22 Interestingly, PMN numbers were elevated in K3n/n and K3n/− mice, albeit to a lesser extent than in K3−/− chimeric mice (Table 1). Although the integrin levels on K3n/n and K3n/− PMNs were normal (data not shown), their kindlin-3 protein content was 10% and 5% of the normal kindlin-3 amount (Figure 4A). To test adhesion to ICAM-1 in vitro, we stimulated PMNs with tumor necrosis factor (TNF)-α or PMA, seeded them on immobilized ICAM-1, and found that K3n/n and K3n/− showed diminished adhesion, whereas adhesion of K3−/− PMNs was abolished, indicating reduced β2 integrin activity in kindlin-3 hypomorphic PMNs (Figure 4B-C). Furthermore, we also measured reduced β1 integrin activity in kindlin-3 hypomorphic PMNs by using the 9EG7 antibody (supplemental Figure 7). To corroborate the relevance of these findings in an in vivo setting, we analyzed leukocyte adhesion quantitatively in TNF-α–stimulated cremaster muscle venules using intravital microscopy. The experiments revealed that leukocyte adhesion was decreased by ∼20% in K3n/n mice and by ∼50% in K3n/− mice (Figure 5A). Consistent with the reduced in vivo adhesion of leukocytes, the numbers of rolling leukocytes in K3n/− mice were increased (Figure 5B).
Kindlin-3 hypomorphic PMNs show impaired adhesion in vitro. (A) Densitometric quantification of kindlin-3 levels in K3+/+, K3+/n, K3n/n, and K3n/− PMNs (N = 3). GAPDH served as loading control. (B) Static adhesion of PMNs to ICAM-1 upon TNF-α stimulation (N = 4). (C) Static adhesion of PMNs to ICAM-1 upon PMA stimulation (N ≥ 5). Data are shown as mean ± SD and were statistically analyzed by Student t test. *P < .05; **P < .01; ***P < .001. n.s., not significant.
Kindlin-3 hypomorphic PMNs show impaired adhesion in vitro. (A) Densitometric quantification of kindlin-3 levels in K3+/+, K3+/n, K3n/n, and K3n/− PMNs (N = 3). GAPDH served as loading control. (B) Static adhesion of PMNs to ICAM-1 upon TNF-α stimulation (N = 4). (C) Static adhesion of PMNs to ICAM-1 upon PMA stimulation (N ≥ 5). Data are shown as mean ± SD and were statistically analyzed by Student t test. *P < .05; **P < .01; ***P < .001. n.s., not significant.
Leukocyte adhesion, extravasation, and bacterial clearance are impaired at low kindlin-3 levels. (A-B) Leukocyte adhesion and rolling in TNF-α–stimulated cremaster muscle venules assessed in 12 venules of 4 K3+/+ mice, in 21 venules from 4 K3+/n mice, in 19 venules of 4 K3n/n mice, and in 17 venules of 3 K3n/− mice. (A) Leukocyte adhesion efficiency determined as the number of adherent leukocytes per mm2 vascular surface area divided by the systemic leukocyte count. (B) Leukocyte rolling flux fraction determined as rolling leukocytes passing an imaginary perpendicular line over the vessel corrected by the total number of passing leukocytes. Values are mean ± SEM. (C-D) Whole-mount immunofluorescence staining of ear flaps at 1 hour (C) and 4 hours (D) after phorbol ester treatment. Tissues were stained with a pan-laminin antibody (LN; green) to visualize endothelial basement membrane and a Gr-1 antibody (red) to visualize neutrophils. The scale bar represents 200 µm. (E) Phagocytosis of fluorescently labeled E coli particles by K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− PMNs (N = 3, 5, 4, 4, 3). Data are shown as mean ± SD and were statistically analyzed by Student t test. (F-H) Infection of kindlin-3 hypomorphic mice with H pylori. (F) Colonization density of gastric mucosa of K3+/+, K3n/n, and K3n/− mice, which were either not infected or were orally infected with H pylori PMSS1 for 12 weeks. (G) Histologic grading of gastric mucosa from K3+/+, K3n/n, and K3n/− mice challenged with H pylori PMSS1 for 12 weeks compared with age-matched noninfected controls. (H) Recruitment of granulocytes to the gastric mucosa of K3+/+, K3n/n, and K3n/− mice challenged with H pylori PMSS1 for 12 weeks compared with age-matched noninfected controls. Data were statistically analyzed by Mann-Whitney U test for unpaired groups. *P < .05; **P < .01; ***P < .001. n.s., not significant.
Leukocyte adhesion, extravasation, and bacterial clearance are impaired at low kindlin-3 levels. (A-B) Leukocyte adhesion and rolling in TNF-α–stimulated cremaster muscle venules assessed in 12 venules of 4 K3+/+ mice, in 21 venules from 4 K3+/n mice, in 19 venules of 4 K3n/n mice, and in 17 venules of 3 K3n/− mice. (A) Leukocyte adhesion efficiency determined as the number of adherent leukocytes per mm2 vascular surface area divided by the systemic leukocyte count. (B) Leukocyte rolling flux fraction determined as rolling leukocytes passing an imaginary perpendicular line over the vessel corrected by the total number of passing leukocytes. Values are mean ± SEM. (C-D) Whole-mount immunofluorescence staining of ear flaps at 1 hour (C) and 4 hours (D) after phorbol ester treatment. Tissues were stained with a pan-laminin antibody (LN; green) to visualize endothelial basement membrane and a Gr-1 antibody (red) to visualize neutrophils. The scale bar represents 200 µm. (E) Phagocytosis of fluorescently labeled E coli particles by K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− PMNs (N = 3, 5, 4, 4, 3). Data are shown as mean ± SD and were statistically analyzed by Student t test. (F-H) Infection of kindlin-3 hypomorphic mice with H pylori. (F) Colonization density of gastric mucosa of K3+/+, K3n/n, and K3n/− mice, which were either not infected or were orally infected with H pylori PMSS1 for 12 weeks. (G) Histologic grading of gastric mucosa from K3+/+, K3n/n, and K3n/− mice challenged with H pylori PMSS1 for 12 weeks compared with age-matched noninfected controls. (H) Recruitment of granulocytes to the gastric mucosa of K3+/+, K3n/n, and K3n/− mice challenged with H pylori PMSS1 for 12 weeks compared with age-matched noninfected controls. Data were statistically analyzed by Mann-Whitney U test for unpaired groups. *P < .05; **P < .01; ***P < .001. n.s., not significant.
To test whether the reduced adhesion leads to reduced extravasation of PMNs, we treated earflaps with phorbol ester (crotoin oil) for 1 hour and observed a delay in the extravasation of K3n/n and K3n/− PMNs compared with wild-type and K3+/n PMNs (Figure 5C), because 4 hours after treatment, K3n/n and K3n/− PMNs had crossed the vascular wall and distributed in the interstitium similar to wild-type PMNs. In contrast, K3−/− PMNs failed to extravasate (Figure 5D).
Next we tested whether the impaired adhesion and extravasation is caused by reduced integrin clustering and/or adhesion signaling in kindlin-3 hypomorphic PMNs. Interestingly, we did not observe differences in αLβ2 and αMβ2 integrin cluster formation upon TNF-α treatment, suggesting that clustering of β2 integrins can proceed in the absence of kindlin-3 (supplemental Figure 8A-B). Moreover, similar to normal FAK phosphorylation in kindlin-3 hypomorphic platelets, a robust β2 integrin–mediated adhesion signaling indicated by p38 and Pyk2 phosphorylation was detected in hypomorphic PMNs, whereas no phosphorylation of these proteins was detected in K3−/− PMNs upon adhesion on ICAM-1 (supplemental Figure 8C).
We then studied the ability of kindlin-3 hypomorphic PMNs to clear bacteria. First, we measured β2 integrin–mediated phagocytosis of serum-opsonized E coli bacteria in vitro. These experiments revealed a stepwise reduction in the bacterial uptake in K3+/n, K3n/n, K3n/−, and K3−/− PMNs, respectively (Figure 5E). To confirm that bacterial colonization clearance is also affected in vivo, we used the H pylori mouse infection model.25 To this end, wild-type, K3n/n, and K3n/− mice were orally infected with the gastric bacterial pathogen H pylori. After chronic infection (3 months), the number of colony-forming units in the stomach, the grade of gastric inflammation, and the number of immigrated PMNs into the gastric tissue was determined (for details see Material and methods). The bacterial load showed a gradual increase in wild-type, K3n/n, and K3n/− mice, indicating that the phagocytic elimination of the bacterial pathogen in vivo was significantly reduced. Gastric inflammation and granulocyte numbers in the gastric tissue were similar in wild-type and K3n/n mice, but were significantly reduced in K3n/− mice (Figure 5F-H).
Altogether, these findings indicate that decreased kindlin-3 levels impair PMN adhesion, extravasation, and bacterial phagocytosis.
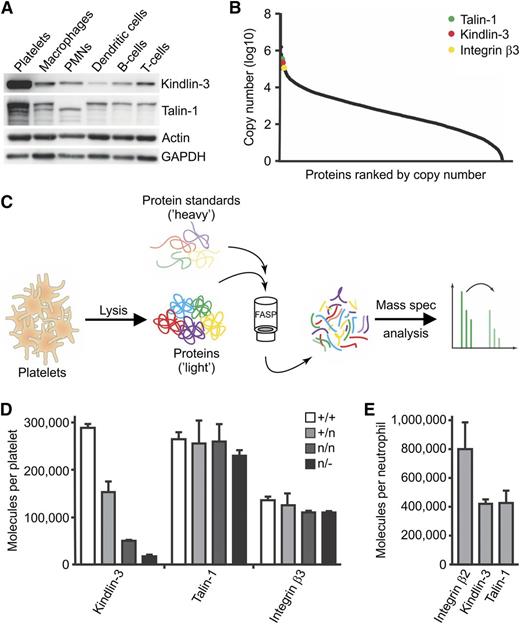
Kindlin-3, talin-1, and integrin copy numbers in platelets and PMNs
Low levels of kindlin-3 are sufficient to execute the basal functions of platelets and PMNs in vivo, whereas they are insufficient to cope with stress situations such as bleeding and infection. Western blot analysis revealed strong differences in the expression of kindlin-3 and talin-1 in different hematopoietic cell populations with highest levels in platelets (Figure 6A). The platelet proteome determined by high-resolution MS26 confirmed that kindlin-3, talin-1, and β3 integrins are among the most abundant platelet proteins (Figure 6B). To determine the exact quantities of kindlin-3, talin-1, and integrins in platelets and PMNs, we used a recently developed MS method to quantify absolute protein numbers by expressing parts of the target protein sequence in a heavy-labeled form.17,27 Specifically, short protein fragments of approximately 150 amino acids from murine kindlin-3, talin-1, and β3 and β2 integrins were fused to the ABP and expressed in the presence of 13C615N2-lysine (Lys8 ) and 13C615N4-arginine (Arg10 ) in an auxotrophic E coli strain to label them with heavy isotopes. A precisely quantified cocktail of different stable isotope–labeled peptide standards from kindlin-3, talin-1, and β2 and β3 integrins was then mixed with platelet and PMN lysates, and their absolute copy numbers per cell were calculated after determining the heavy-to-light ratio by MS (Figure 6C; for details see Material and methods and Zeiler et al17 ). Kindlin-3 expression in platelet lysates from wild-type, K3+/n, K3n/n, and K3n/− mice revealed a gradual decrease, whereas talin-1 and β3 integrin levels remained unchanged in the different platelet populations (Figure 6D). The measurements also revealed that a single wild-type platelet contained approximately 260 000 and 290 000 copies of talin-1 and kindlin-3, respectively, and about 130 000 copies of β3 integrin molecules (Figure 6D). Wild-type PMNs also contained stoichiometric quantities of kindlin-3 and talin-1, with around 420 000 molecules per cell, whereas the total number of β2 integrins was approximately 800 000 molecules per cell (Figure 6E). These data indicate that platelets contain an excess of absolute numbers of talin-1 and kindlin-3 molecules over αIIbβ3 integrins, whereas the relationship with β2 integrins is reversed in PMNs.
Quantification of kindlin-3, talin-1, and integrin protein levels in platelets and PMNs. (A) Western blot of kindlin-3 and talin-1 from different blood cell lysates. GAPDH and actin served as loading controls. (B) Copy number counts of platelet proteins were estimated with intensity-based absolute quantification. (C) Schematic representation of the workflow for quantification of protein copy numbers in platelet lysates. Heavy-labeled protein standards with known concentration were spiked into platelet lysates, and peptide ratios were determined by mass spectrometry. (D) Mass spectrometry–based absolute quantification of the copy numbers of kindlin-3, talin-1, and β3 integrin in K3+/+, K3+/n, K3n/n, and K3n/− platelets (N = 3, 2, 2, 2). Values are given as median ± range. (E) Mass spectrometry–based absolute quantification of the copy numbers of kindlin-3, talin-1, and β2 integrin in wild-type PMNs (N = 3). Values are given as median ± range.
Quantification of kindlin-3, talin-1, and integrin protein levels in platelets and PMNs. (A) Western blot of kindlin-3 and talin-1 from different blood cell lysates. GAPDH and actin served as loading controls. (B) Copy number counts of platelet proteins were estimated with intensity-based absolute quantification. (C) Schematic representation of the workflow for quantification of protein copy numbers in platelet lysates. Heavy-labeled protein standards with known concentration were spiked into platelet lysates, and peptide ratios were determined by mass spectrometry. (D) Mass spectrometry–based absolute quantification of the copy numbers of kindlin-3, talin-1, and β3 integrin in K3+/+, K3+/n, K3n/n, and K3n/− platelets (N = 3, 2, 2, 2). Values are given as median ± range. (E) Mass spectrometry–based absolute quantification of the copy numbers of kindlin-3, talin-1, and β2 integrin in wild-type PMNs (N = 3). Values are given as median ± range.
Discussion
The findings of the present study show that 5% of physiologic kindlin-3 levels in platelets and PMNs compromise their integrin functions but still prevent spontaneous bleeding and lethality, which are key phenotypes that occur in the absence of kindlin-3 in mouse and human.7-11,22 The observation that with very low levels of kindlin-3, still sufficient numbers of integrin/kindlin-3 complexes are formed to sustain basal in vivo functions is consistent with results obtained with mutagenesis studies, in which the interaction of kindlin-3 with integrin β tails was manipulated. Such experiments massively decrease but do not necessarily eliminate kindlin-3–integrin interactions.28,29 Under these circumstances, the residual integrin activity is sufficient to prevent severe spontaneous defects, which develop when protein expression is completely lost or the protein-protein interaction is fully abolished.
It is surprising that as little as 5% of physiologic kindlin-3 levels are sufficient to prevent spontaneous bleeding and microbial infections. However, hemostasis or control of bacterial colonization fails under stress conditions such as tail wounding or chronic exposure to H pylori. This finding strongly suggests that the majority of surface integrins and integrin regulators serve as a backup system that is activated to save the organism in life-threatening situations. Indeed, studies on leukocyte adhesion deficiency type I patients showed that the severity of infectious complications is related to the degree of β2-integrin deficiency. Leukocyte adhesion deficiency type I patients with severe immune deficiency have essentially undetectable β2-integrin expression on leukocytes, whereas moderate phenotypes are found in patients expressing 2.5% to 6% of β2 integrins on their neutrophils.30,31
We also found stoichiometric quantities of kindlin-3 and talin-1 in platelets and PMNs, supporting the current opinion that the 2 proteins intimately cooperate with each other. Although absolute quantities of talin-1, kindlin-3, and integrin molecules are higher in PMNs than in platelets, it has to be considered that platelets have a much smaller surface area and volume compared with PMNs, which leads to a higher density and concentration of integrins and their activators in platelets than in PMNs. The high density of active integrins is probably required to rapidly convert platelets from free flowing to an instant arrest state on injured vessel surfaces. Subsequently, the generation of thrombin at the site of injury and other soluble agonists, such as adenosine diphosphate and thromboxane A2, will further drive platelet activation, leading to the formation of a densely packed platelet core within a growing thrombus.32 In contrast, PMNs roll over a distance of several hundred micrometers until sufficient integrins are activated to induce their arrest, crawling, and extravasation.33
Our quantitative measurements also revealed a twofold excess of talin-1 and kindlin-3 numbers over β3 integrins in platelets, whereas neutrophils contain twice as much β2 integrins than talin-1 and kindlin-3. Platelets activate all their integrins at the same time to achieve instant arrests and stable clots, which is guaranteed by an excess of talin-1 and kindlin-3. Conversely, neutrophils express a variety of integrins with distinct functions that are not necessarily required at the same time, but rather consecutively.34-36 Notably, initial neutrophil adhesion to the endothelium is achieved with the constitutively expressed β2 integrins (αLβ2 and αMβ2 integrins), whereas the majority of β2 integrins (primarily αMβ2 integrins), however, is stored in intracellular vesicles, exocytosed during neutrophil rolling and arrest, and required for their crawling on activated endothelial cells.35,37,38 Neutrophil crawling is highly dynamic and requires the activation of a restricted number of αMβ2 integrins to enable a cyclic adhesion/de-adhesion process with the endothelium and subendothelial tissue. The activation of β2-integrin subsets on neutrophils to enable a series of consecutive and dynamic processes is therefore still guaranteed with lower numbers of talin-1 and kindlin-3 molecules relative to β2 integrins in neutrophils.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michal Grzejszczyk, Susanne Bierschenk, and Eva Löll for excellent technical assistance; and Kevin Flynn, Karim Dib, Roy Zent, and Ambra Pozzi for critically reading the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 914 TP A01/A05/B01/B05) and the Max Planck Society.
Authorship
Contribution: S.K. designed and performed most of the experiments and analyzed data; F.A.M., M.Z. R.R., and U.B. designed and performed experiments and analyzed data; S.M. analyzed data; R.H. designed research and analyzed data; M. Mann provided essential instrumentation; M.S. designed and performed experiments, analyzed data, and contributed to the discussion; R.F. designed and supervised research and wrote the paper; and M. Moser designed and performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Moser, Max-Planck-Institute of Biochemistry, Am Klopferspitz 18, D-82152 Martinsried, Germany; e-mail: moser@biochem.mpg.de.


![Figure 2. Function of platelet integrins is kindlin-3 dose-dependent. (A) Densitometric quantification of kindlin-3 levels in K3+/+, K3+/n, K3n/n, and K3n/− platelets (N = 5, 5, 4, 4). GAPDH served as loading control. (B) K3+/+, K3+/n, K3n/n, K3n/− E14.5 embryos are free of bleeding. (C) Tail bleeding times of K3+/+, K3+/n, K3n/n, and K3−/− chimeric mice. (D-E) Binding of fibrinogen to platelets after treatment with 0.1 U/mL (D) and 1 U/mL thrombin (E). Resting platelets were used as control (N = 9, 8, 8, 10, 6 [D] and N = 8, 3, 8, 8, 2 [E]). (F-G) Activation of αIIbβ3 integrin on platelets upon stimulation with 0.1 U/mL (F) and 1 U/mL (G) thrombin (N = 13, 8, 11, 16, 5 [F] and N = 15, 10, 16, 16, 4 [G]). (H) β1 integrin activation index of K3+/+, K3+/n, K3n/n, K3n/−, and K3−/− platelets upon activation with 1 U/mL thrombin (N = 10, 7, 10, 8, 3). (I) Activation of αIIbβ3 integrin on control, kindlin-3−/−, talin-1−/−, and integrin β3−/− platelets upon stimulation with 0.1 U/mL and 1 U/mL thrombin (N = 6, 6, 6, 3). (J) Western blot analyses of talin-1 and kindlin-3 expression in lysates from wild-type (+/+), K3−/−, and talin-1−/− platelets. GAPDH served as loading control. (K) Platelet aggregation assays with +/+, K3−/−, and talin-1−/− platelets in response to 0.1 U/mL thrombin. Values are given as mean ± SD and data were statistically analyzed by Student t test. *P < .05; **P < .01; ***P < .001. n.s., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/24/10.1182_blood-2015-04-639310/4/m_2592f2.jpeg?Expires=1767698710&Signature=mHXAzBkVnrOqk2hiI4iFw0utqho~yBa-REb5jHPw73TXRDdQgkNobvDvoWdJQrHhn3qPa3pwIr71x~y-vo6MFg1Cae6l6HNzRSWPg0dQjmFkSfHhZaDk1lTn6Pa8yB3qkejGbDitnuQTHSkVJAYwU96gGRXt3y34RyaWnbv6LzkGDuluHzkfCtYHDSB3lbxQuz5qFlwuHRCUBxzmdK8sKwnmmQomS8Il-Yd26UAyeQ2H1yWoFHilIJSJp17tYhxn10w0lmZNxNTCPV53QIzX7b8-hH9z1ufdPYieQPl-zEsNvZkBIDSVPNasxEuCfG96Gn~668d0xXt5QGzMG5FV1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)