In this issue of Blood, Klapproth et al used mice that were hypomorphic for the protein kindlin-3 to demonstrate that as little as 5% of the normal amount of kindlin-3 is sufficient to protect against spontaneous bleeding and infection.1
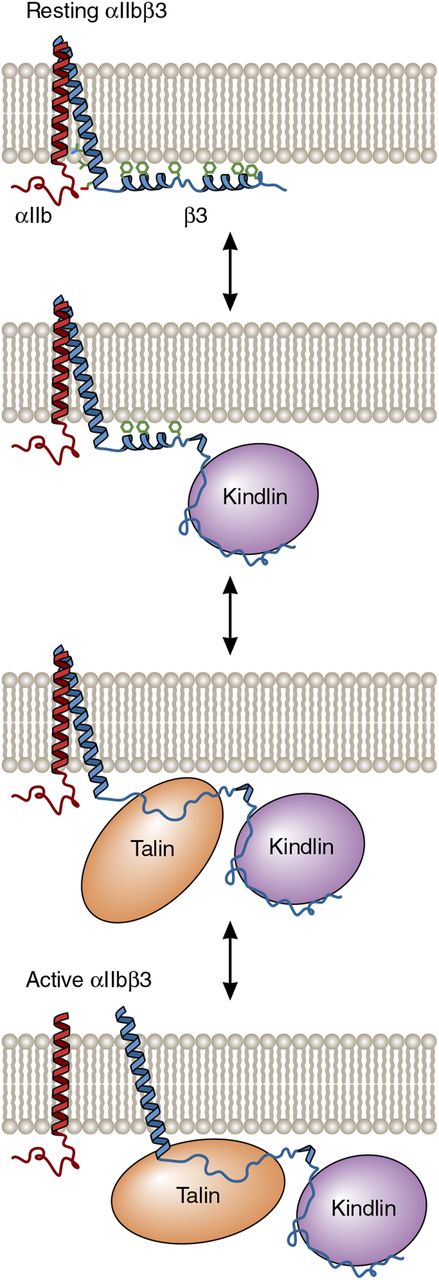
One possible model for the cooperative activation of αIIbβ3 by kindlin-3 and talin-1. In resting platelets, the 2 distal helices of the β3 cytoplasmic tail interact with the membrane bilayer and are not competent to bind talin-1 or kindlin-3 because the membrane obstructs their access to β3. However, the dynamic nature of the tail makes protein-protein interaction motifs accessible. As depicted in the model, kindlin-3 binding to the distal β3 tail removes the proximal tail from the membrane, thereby allowing it to bind to the talin-1 FERM domain. In turn, talin-1 FERM domain binding causes separation of the αIIbβ3 transmembrane domain heterodimer and subsequent αIIbβ3 activation. Adapted from Metcalf et al6 with permission. Professional illustration by Patrick Lane, ScEYEnce Studios.
One possible model for the cooperative activation of αIIbβ3 by kindlin-3 and talin-1. In resting platelets, the 2 distal helices of the β3 cytoplasmic tail interact with the membrane bilayer and are not competent to bind talin-1 or kindlin-3 because the membrane obstructs their access to β3. However, the dynamic nature of the tail makes protein-protein interaction motifs accessible. As depicted in the model, kindlin-3 binding to the distal β3 tail removes the proximal tail from the membrane, thereby allowing it to bind to the talin-1 FERM domain. In turn, talin-1 FERM domain binding causes separation of the αIIbβ3 transmembrane domain heterodimer and subsequent αIIbβ3 activation. Adapted from Metcalf et al6 with permission. Professional illustration by Patrick Lane, ScEYEnce Studios.
The β2 and β3 integrins on circulating leukocytes and platelets, respectively, are maintained in inactive states, unable to bind to their respective ligands, to prevent spontaneous cellular adhesion and cohesion. However, after bacterial infection or vascular injury, leukocyte and platelet integrins are rapidly activated to enable fast leukocyte adhesion, extravasation, and bacterial clearance, as well as effective platelet plug formation. In agonist-stimulated leukocytes and platelets, integrin activation occurs when the FERM (4.1/ezrin/radixin/moesin) domains of the cytoskeletal protein talin-1 and the focal adhesion protein kindlin-3 interact with the cytoplasmic tails of β2 and β3, respectively. This causes transmembrane domain and extracellular stalk separation and opening of extracellular domain ligand binding sites.2 In vitro, the talin-1 FERM domain alone appears sufficient to cause the activation of purified platelet integrin αIIbβ3, whereas kindlin-3 does not.3,4 In vivo, however, kindlin-3 deficiency impairs leukocyte adhesion and extravasation and produces a platelet phenotype reminiscent of Glanzmann thrombasthenia, despite the presence of normal amounts of talin.5 Thus, both talin and kindlin are required for physiologic integrin activation in leukocytes and platelets, but why both proteins are needed remains unclear. One possibility, based on a nuclear magnetic resonance structure of the β3 cytoplasmic tail and its interaction with membrane bilayers as determined by hydrogen-deuterium exchange, posits that kindlin binding to the distal β3 tail exposes and stabilizes a more proximal talin binding site, enabling talin binding to disrupt the αIIbβ3 transmembrane heterodimer (see figure).6 This model suggests that kindlin might bind simultaneously with talin in a positively cooperative manner, although it has not yet been possible to trap the putative ternary complex. Thus, it is possible that kindlin acts transiently to increase the accessibility of the talin binding site. Yet another possibility is that talin instead binds first, and subsequent kindlin recruitment induces integrin clustering and increased integrin avidity for multivalent ligands such as fibrinogen.4
In the study by Klapproth et al,1 the authors asked whether granulocyte and platelet function require a threshold level of kindlin-3, taking advantage of their observation that introducing a neomycin cassette into intron 6 of the murine kindlin-3 gene diminished its expression. By selective breeding, they then produced mice whose platelets and granulocytes contained 5%, 10%, and 50% of the wild-type (WT) amount of kindlin-3. Kindlin-3–null mice die shortly after birth from severe anemia caused by bleeding and red-cell defects. Thus, it was surprising that mice expressing as little as 5% of the WT amount of kindlin-3 were born at the expected Mendelian ratios, developed and aged normally, and were not anemic. Nonetheless, the 10% and 5% mice had markedly prolonged bleeding times, and there was progressive decrement in laboratory measurements of platelet integrin function as kindlin-3 levels decreased below 50%. These observations indicate that 5% to 10% of the WT amount of platelet kindlin-3 is sufficient to support basal hemostatic function but is not sufficient to support platelet function when mice are exposed to hemostatic stress.
Like kindlin-3–deficient platelets, granulocytes from the 10% and 5% mice adhered poorly to the αLβ2 ligand intercellular adhesion molecule-1, and there was delayed granulocyte extravasation into inflamed interstitium, a finding consistent with their mild granulocytosis. Furthermore, there was a stepwise decrease in bacterial phagocytosis as the amount of granulocyte kindlin-3 decreased along with decreased bacterial clearance in a chronic Helicobacter pylori gastric infection model. It is noteworthy, however, in view of the possible mechanisms of kindlin activity discussed above, that αLβ2 and αMβ2 clustering appeared to be unaffected in kindlin-3–deficient granulocytes.
The authors also compared the amounts of platelet and granulocyte talin-1, kindlin-3, and integrin in the various kindlin-3-deficient mice by using a novel mass spectrometry–based method. They found that decreasing the amount of kindlin-3 in platelets did not affect the amount of talin-1 or β3 integrin and that kindlin-3 and talin-1 were present in essentially stoichiometric amounts in WT cells. They also found approximately twice as much kindlin-3 and talin-1 as integrin in platelets, whereas the opposite was the case for granulocytes, a finding that may contribute to differences in integrin activation kinetics in these cells.
The results reported by Klapproth et al1 demonstrate that despite the presence of talin-1 concentrations sufficient to saturate its β-integrin binding sites, integrin function in platelets and granulocytes can be titrated by varying the amount of kindlin-3. This is consistent with the idea that kindlin-3 plays a permissive role in enabling talin to activate integrins. They also found that relatively little active integrin is required to support basal platelet and granulocyte function, perhaps explaining the surprisingly mild bleeding diathesis (in the absence of stress) of many adult patients with Glanzmann thrombasthenia7 and the relatively mild infectious complications experienced by some patients with leukocyte adhesion deficiency III.8 Despite the insights into integrin activation provided by these studies, important questions remain about the dual roles for talin and kindlin in regulating integrin function. In resting platelets, the integrin-binding site in the talin-1 FERM domain is masked by sequences located in the talin-1 rod domain.9 But there is no clear explanation of how platelet stimulation relieves this apparent talin-1 autoinhibition. Kindlins are structurally similar to talin FERM domains but have a lipid-binding Pleckstrin homology (PH) domain inserted into their F2 subdomain and, in contrast to talins, they are not autoinhibited.10 However, as with talin-1, it is unclear how agonist stimulation causes kindlin binding to β-integrin tails. Kindlins bind to lipids as well as integrins, and the PH domain of kindlin-2, a kindlin family member more widely expressed than kindlin-3 whose expression is limited to hematopoietic cells, binds to membrane phosphoinositides.11 Thus, it is possible that in platelets and granulocytes, agonist-stimulated phosphorylation of membrane phosphoinositides drives kindlin to the plasma membrane where it encounters a high density of integrin cytoplasmic tails.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal