In this issue of Blood, Katsuya et al report on the clinical data of 1594 patients collected retrospectively from medical records of patients diagnosed with adult T-cell leukemia (ATL) between 2000 and 2009 in Japan (ie, the ATL Prognostic Index project).1 This is the largest study elucidating practical management and outcome for ATL patients treated by conventional chemotherapy and allogeneous hematopoietic stem cell transplantation (allo-HSCT). In comparison with the first Japanese survey performed between 1983 and 1987, this study reveals that although response rates to chemotherapy have increased, overall survival (OS) remains poor and virtually only patients who could undergo allo-HSCT experience long-term survival.
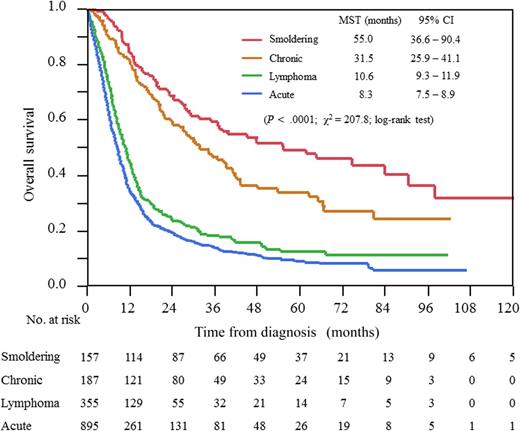
OS according to ATL subtypes (Shimoyama classification). CI, confidence interval; MST, median survival time. See Figure 2 in the article by Katsuya et al that begins on page 2570.
OS according to ATL subtypes (Shimoyama classification). CI, confidence interval; MST, median survival time. See Figure 2 in the article by Katsuya et al that begins on page 2570.
ATL is the first human malignancy associated with chronic infection by the human T-cell lymphotropic virus type 1 (HTLV-1) retrovirus. ATL occurs after a long latency period in about 5% of 10 to 20 million infected people in the world. HTLV-1 infection appears to represent the first event of a multistep oncogenic process.2 After infection, the virus is integrated in the host genome and the viral transactivator protein Tax is expressed and interacts with many cellular pathways that regulate apoptosis, proliferation, and activation, and is involved in DNA repair and epigenetic regulation, resulting in oligoclonal expansions of HTLV-1–infected T cells. Although there is no hotspot of virus integration, experimental data suggest that integration may deregulate host genes involved in the pathophysiology of other leukemia/lymphomas. In most of the cases, the proliferation of these clones is controlled by the immune system. Secondary genetic events may be responsible for transformation into overt leukemia or lymphoma. They include mutation or deletion that overlap significantly with the HTLV-1 Tax interactome,3 allowing cells to proliferate without requiring Tax expression anymore and thus escape immunosurveillance. Mutations or deletions of tumor suppressor genes such as p53 or p15INK4B/p16INK4A are found in 30% to 50% of ATL cases and are associated mostly with clinical subtypes with a poor prognosis. In contrast to Tax protein, the expression of the HTLV-1 bZIP factor gene (HBZ), an antisense messenger RNA transcribed from the 3′ long terminal repeat of the virus, has been shown to be consistently present in ATL cells, but its precise role in lymphomagenesis remains to be fully elucidated.2 Adding to the complexity of ATL lymphomagenesis, several clones with different genetic signatures may co-exist or succeed each other with time and in different organs invaded by the tumor cells.2
ATL carries a very poor prognosis because of intrinsic chemoresistance and severe immunosuppression. The presentation and course of the disease are heterogeneous. In the first description in 1991, Shimoyama described 4 clinical forms of ATL (acute, lymphoma, chronic, and smoldering) depending on blood involvement, the presence of circulating cells, and biological parameters, including hypercalcemia and lactate dehydrogenase levels.4 In the aggressive forms (acute and lymphoma), clinical trials mainly conducted in Japan showed that combinations of chemotherapy can induce acceptable complete response (CR) rates in ATL lymphoma (40% to 60%) but to a lower rate in the acute form (30%). However, due to a high relapse rate in both forms, long-term prognosis was poor (OS, 25% at 2 years). For responding patients, autologous SCT is not a good option because it is associated with a high risk of infection and relapse.5 Several nonrandomized studies have shown that the only way to cure ATL is allo-SCT (OS, 30% to 45%).6 However, the number of ATL patients eligible for allo-SCT is very limited because of the low CR rate (especially in the acute form), poor performance status, severe immunosuppression, and low probability of finding a suitable donor in patients from ethnic migrant minorities outside Japan.
For the so-called indolent forms (smoldering and chronic ATL) considered as having a good prognosis in the first Shimoyama classification, a recent report has suggested that in fact they also have a poor long-term prognosis if they are managed with a watchful waiting policy, which was even worse when chemotherapy was used.7
In the study reported in this issue,1 Katsuya et al reported the prognosis of ATL treated by conventional chemotherapy and allo-HSCT from “real-life” patients of several Japanese centers (see figure). They show that among acute and lymphoma type ATLs, various multiagent chemotherapy regimens were used without a clear superiority of any regimen to increase OS. For patients with aggressive ATL, they confirm that allo-HSCT is the only option to cure aggressive ATL, when they are in CR after chemotherapy. However, the 3-year OS was still low at only ∼35%, and allo-HCST caused high transplant-related mortality of up to or more than 40% and was therefore not applicable to all patients. They also confirm that patients with chronic types also have a poor prognosis with <50% of patients alive at 5 years. Therefore, this report suggests that conventional chemotherapies have reached their limits to cure patients in all forms of ATLs, and thus new therapeutic strategies are eagerly awaited.
In Japan, no patients with acute and lymphoma type ATLs received antiretroviral therapy by interferon and zidovudine (IFN/AZT) because of the lack of approval of both agents for the treatment of ATL under the national health insurance system. This therapeutic combination may be effective in the leukemic forms of ATL (OS, 30% at 5 years), particularly in the group of patients without p53 mutations as well as in patients with indolent forms (OS, >90% at 5 years), and long-term survivors are observed even without allo-SCT. In contrast, INF/AZT was not effective in the lymphoma subgroup.8 Thus, INF/AZT is now considered as standard first-line therapy in some parts of Europe, the Middle East, and the United States5 and is currently being evaluated in Japan in a randomized study against a watch and wait policy in patients with indolent forms of ATLs.
In the future, important questions remain to be answered. Should we (1) decide on treatment based on the Shimoyama classification and/or on a molecular basis? (2) Use drugs approved or in development in other T-cell lymphomas, or (3) take advantage of the virus infection and/or knowledge of molecular events to define new therapeutic strategies? Performing clinical randomized studies testing new drugs will be a difficult task because of the small number of patients outside Japan. It is also highly probable, because of the high heterogeneity of T-cell clones between patients and even within the same patient, that no “universal” treatment may treat all ATLs. However, with advances in tumor genotyping, personalized targeted therapy with specific antibodies (such as anti-CCR4) and/or small molecules should be tested alone or in combination with conventional chemotherapies. Studies with INF/arsenic have shown an increase in time to relapse,9 which may be due to the destruction of Tax-expressing leukemia initiating cells as a result of Tax proteasome degradation.10 Vaccine strategies (curative and preventive) against virus proteins like Tax or HBZ, alone or associated with immune checkpoint inhibitors, could be also interesting options in the future that target the “virus.”
We are now at an opportune time to evaluate new therapeutic strategies based on our knowledge of ATL lymphomagenesis. The tremendous work of Japanese investigators reported by Katsuya et al will serve as a new starting point in ATL treatment. However, because outside of Japan ATL occurs in areas of the world where access to health care is limited, the development of therapeutic strategies should take this limitation into account. In this regard, treatment with antiviral therapy (INF/AZT, arsenic, and others) and vaccines should be considered. Meanwhile, new approaches preventing transmission through breast-feeding, a major route of infection (associated with a risk of ATL development), need to be developed.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal