Abstract
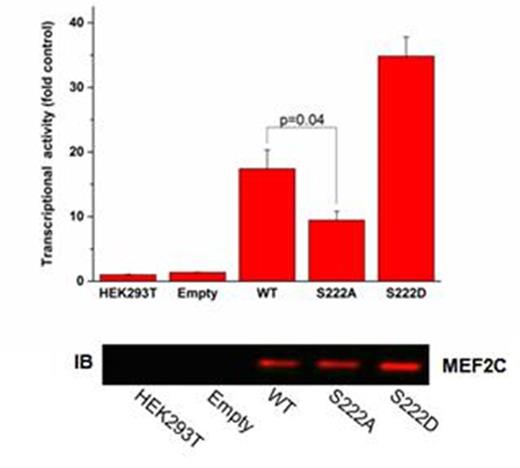
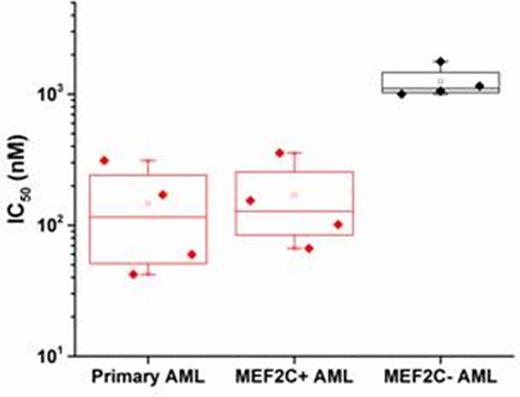
Despite intense efforts, the cure rates of children and adults with AML remain unsatisfactory. Resistance to cytotoxic chemotherapy is the dominant cause of treatment failure. To investigate the molecular basis of primary chemoresistance, we used targeted gene sequencing using the Foundation One Heme platform to profile 405 genes in DNA and 265 genes in RNA known to be somatically mutated in hematologic malignancies in 107 primary specimens obtained at diagnosis from children and adults with cytogenetically normal AML. Comparative analysis revealed mutations of SETBP1 and ASXL1 to be enriched in patients with failure of induction chemotherapy as compared to those who achieved remission (8 out of 50 versus 1 out of 57, p = 0.01). However, the low prevalence of these alterations indicated that other genomic or functional mechanisms must mediate chemoresistance. To identify these mechanisms, we mapped kinase signaling cascades activated in chemoresistant AML cells using functional mass spectrometry proteomics. Phosphopeptides were enriched in primary leukemic blasts isolated from cytogenetically normal AML patients, with relative abundances in each patient determined from iTRAQ 8-plex reporter ions in the associated MS/MS fragment ion spectra. This analysis identified phosphorylation of the leukemogenic transcription factor MEF2C at S222 in the majority of cases. We found MEF2C to be overexpressed in cytogenetically normal AML, as well as MLL-rearranged leukemias, and to be associated with increased risk of relapse (Laszlo et al, ASH submitted). Using reporter assays, we found that phosphorylation of MEF2C S222 was both necessary and sufficient to fully transactivate MEF2C promoter elements (Fig. 1). Expression of mutant S222A MEF2C that cannot be phosphorylated, but not phosphomimetic S222D, induced mitochondrial apoptosis in human AML and genetically-engineered MLL-AF9 mouse leukemia but not in normal mouse hematopoietic progenitor cells. In syngeneic mouse transplant models, we found that MEF2C phosphorylation was required for the maintenance of MLL-AF9 leukemias in vivo. Transcriptome and proteome analysis of gene expression changes and composition of MEF2C transcriptional complexes induced by phosphorylation mutants showed that aberrant leukemia cell survival is caused at least in part by alterations in the expression of apoptotic regulators. Using a recombinant kinase screen, we identified MARK kinases as specific enzymes that phosphorylate MEF2C S222. Treatment of primary patient specimens and human AML cell lines with the MARK kinase inhibitor MRT199665 induced MEF2C downregulation and apoptosis in cells with MEF2C but not those lacking MEF2C expression (Fig. 2). Thus, aberrant MEF2C phosphorylation is associated with primary chemoresistance and induction failure, is required for enhanced leukemia cell survival, and confers susceptibility to MARK inhibition therapy. In addition, genome and proteome profiles should provide additional biomarkers and targeted therapeutic strategies to overcome chemoresistance in AML.
MEF2C phosphorylation is required for transactivation of MEF2C promoter elements
MEF2C phosphorylation is required for transactivation of MEF2C promoter elements
He:Foundation Medicine, Inc.: Employment, Equity Ownership. Balasubramanian:Foundation Medicine, Inc.: Employment. Zhong:Foundation Medicine, Inc.: Employment, Equity Ownership. Pavlick:Foundation Medicine, Inc.: Employment. Yilmazel:Foundation Medicine, Inc.: Employment. Stone:Merck: Consultancy; AROG: Consultancy; Abbvie: Consultancy; Juno: Consultancy; Amgen: Consultancy; Celator: Consultancy; Agios: Consultancy; Karyopharm: Consultancy; Pfizer: Consultancy; Roche/Genetech: Consultancy; Sunesis: Consultancy, Other: DSMB for clinical trial; Celgene: Consultancy; Novartis: Research Funding. Byrd:Acerta Pharma BV: Research Funding. Levine:CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Foundation Medicine: Consultancy; Loxo Oncology: Membership on an entity's Board of Directors or advisory committees. Armstrong:Epizyme, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal