Abstract
BACKGROUND: The prognosis for patients (pts) with hematologic malignancies (HM) who relapse after alloHSCT is dismal, and novel treatment options are urgently needed. Checkpoint blockade has the potential to augment the graft-vs-tumor (GVT) effect pharmacologically, but may increase the risk of graft-vs-host disease (GVHD). We conducted a multicenter, open-label phase I/Ib study of the CTLA4 blocking antibody ipilimumab in pts with relapsed HM after alloHSCT.
METHODS: The primary objectives were to determine the MTD and evaluate safety. Secondary objectives were to assess efficacy and changes in circulating lymphocyte populations. Ipilimumab induction was given IV every 3 weeks for 4 cycles, followed by maintenance dosing every 12 weeks up to 1 year. Disease-specific response evaluations were performed at the mid-point (7 weeks), end of induction (13 weeks), and throughout maintenance. Immunophenotyping was assessed by 8-color flow cytometry. Two dose levels (3 mg/kg and 10 mg/kg) were studied in phase I. The 10 mg/kg dose was chosen for expansion on the basis of safety and efficacy results.
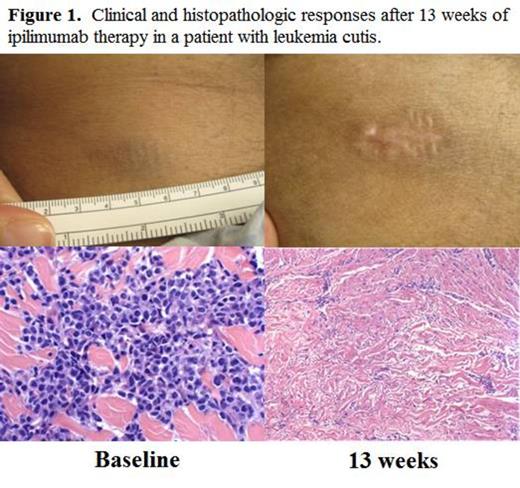
RESULTS: A total of 28 patients were enrolled. In phase I, 6 pts were treated at 3 mg/kg and 7 pts were treated at 10 mg/kg. In the phase Ib expansion cohort, 15 additional pts were treated at 10 mg/kg. Histologies included AML (n=12, including 3 pts with leukemia cutis), HL (n=7), NHL (n=4), and MDS (n=2), and 1 pt each had MM, MPN, and ALL. The median age at enrollment was 58 yrs. (range 22-75). The median number of prior therapies excluding transplant was 3 (range 2-11), and 19/27 (70%) of pts had received prior therapy for post transplant relapse. In the toxicity analysis, immune-related adverse events (irAEs) typical of ipilimumab were observed in 4 pts, including pneumonitis (n=1 gr2, n=2 gr4), diarrhea (n=2 gr 1), colitis (n=1 gr 3), and ITP (n=1 gr2). These irAEs were generally reversible with steroids, and 3 pts were able to resume ipilimumab. Five DLTs leading to discontinuation occured, including cGVHD (n=3, all liver, gr 3), aGVHD (n=1, gut, gr 2), and TRM (n=1) due to presumed sepsis in the context of gr4 pneumonitis and gr3 colitis. Seventeen pts discontinued due to progressive disease, and 6 pts remain on active treatment. In the efficacy analysis, none of the 5 evaluable pts treated at 3 mg/kg achieved a formal response by disease specific criteria. In the 21 evaluable patients treated at 10 mg/kg, 10 patients (48%) had disease reduction. The overall response rate (ORR) by formal disease specific criteria was 33%. Responders included a HL pt with a PR with dramatic reduction in widespread bony disease and a marrow CR by 7 weeks, and a MM pt with a highly refractory lung plasmacytoma who achieved a PR. Notably, 5/12 (42%) pts with AML achieved CR, including all 3 pts with leukemia cutis (see example in Fig. 1) and one pt with myeloid sarcoma. The responses in two of the three leukemia cutis pts were durable, with both pts still in CR at 6 and 8 months on study. With a median follow-up time among survivors of 8.5 mo., the 6 mo. OS is 67%. Immunophenotyping studies revealed that the absolute numbers of T cells (including CD3+, CD4+, CD8+, Tcon, and Treg) peaked at 4 weeks after initial dosing and trended back down by 8 weeks. The ratio of Treg/Tcon decreased between 24 to 41% in the 15 pts with samples available at each time point. In the 5 pts who achieved CR, the Treg/Tcon ratio was similar to the rest of the cohort at baseline (0.07 vs. 0.08, p=0.57), but by 8 weeks there was a trend toward a decreased ratio in CR pts (0.05 vs. 0.15, p=0.14). The absolute number of NK cells was lower in responders than in non-responders by the 8 week time point (p = 0.04).
CONCLUSION: Repeated dosing of ipilimumab is feasible in pts with relapsed HM after alloHSCT, although at 10 mg/kg both irAEs and GVHD were observed on treatment. Ipilimumab in this setting can provide substantial anti-tumor activity in pts with both lymphoid and myeloid malignanices. Interestingly, chemorefractory extramedullary myeloid diseases such as leukemia cutis and myeloid sarcoma appeared to respond particularly well. The Treg/Tconv cell ratio decreased with treatment, consistent with enhancement of GVT. Checkpoint blockade is a promising new therapeutic approach for post alloHSCT relapse, and is worthy of exploration in future studies.
Davids:Pharmacyclics: Consultancy; Janssen: Consultancy; Genentech: Other: ad board. Chen:Bayer: Consultancy, Research Funding. Armand:Sequenta, Inc.: Research Funding; Merck: Consultancy, Research Funding; BMS: Research Funding; Infinity: Consultancy, Research Funding. Soiffer:Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal