Abstract
Introduction: The impact of biological variables on treatment outcomes for patients with multiple myeloma (MM) are key to a stratified medicine approach. Whether to re-start therapy at relapse on serological vs symptomatic progression is an important clinical question. Though salvage autologous transplantation (ASCT2) in MM has been shown to induce superior durability of responses over low-dose alkylating consolidation therapy in the relapsed setting (ISRCTN601231201), who benefits most from this strategy remains to be defined. An important subgroup comparison in Myeloma X was to evaluate the impact of age & disease stage on outcomes after ASCT2 vs non-transplant consolidation (NTC).
Patients and Methods: Eligible patients with MM relapsing after prior ASCT were enrolled. All patients received Bortezomib, Doxorubicin & Dexamethasone (PAD) prior to 1:1 randomization between ASCT2 or NTC with weekly cyclophosphamide for 12 weeks. Response was assessed (by IMWG criteria) after re-induction & 100 days post-randomization. Patients were stratified by β2M at trial entry & ASCT1 time-to-progression (TTP). The 10 endpoint was TTP. Overall survival (OS) was a key 20 endpoint with subgroup analysis of stage, age, symptomatic status & cytogenetic risk.
Results: 297 patients were entered & 174 randomized: ASCT2 n=89, NTC n=85. Median age was 61 (range 38-75) with 22% of patients >65 years. The median TTP from ASCT1 was 31 months (range 8-149) with no impact in terms of age at trial entry. 43% of patients were deemed to have symptomatic relapse (sRel) at trial entry based on CRAB criteria (anaemia 30%, renal disease 16% and hypercalcaemia 5%). There was no significant difference in the median ASCT1 TTP between trial entrants with sRel compared to asymptomatic relapse (aRel: 35 months [95%CI 32,38] vs sRel: 36 months [95%CI 32,40]; Mann-Whitney p=0.657).
Post-randomization, sCR/CR rate was significantly higher after ASCT2 (odds ratio (OR) = 0.42 [95%CI 0.21,0.85]; p=0á012), with no significant age effect identified (likelihood ratio test (LRT) p=0.131). The impact of aRel compared to SRel demonstrated a non-significant trend towards benefit in sCR/CR rate for aRel patients (aRel: OR=0.28 [95%CI 0.11,0.76] vs sRel: OR=0.66 [95%CI 0.23,1.93]; LRT p=0.343).
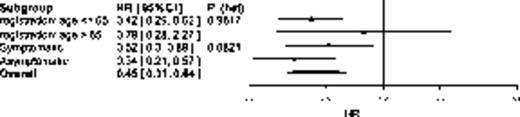
The median follow-up is 52 months (IQR 41, 62) & updated TTP demonstrates continued advantage of ASCT2 over NTC (hazard ratio (HR)=0.45 [95%CI 0.31,0.64]; p<0.0001). Age was not found to have an independent impact on TTP. However, when the impact of aRel was compared with sRel, a non-significant trend towards benefit was demonstrable (aRel: HR=0.34 [95%CI 0.21,0.57]; sRel: HR=0.52 [95%CI 0.30,0.89]; LRT p=0.082) (Fig 1a).
Following progression, 88.7% in the ASCT2 and 84.0% in the NTC cohorts have received 3rd line therapy (primarily lenalidomide-based). The impact of both age at trial entry (²65 yrs: HR=0.36 [95%CI 0.22,0.58]; >65 yrs: HR=1.02 [95%CI 0.30,3.52]; LRT p=0.827) & symptomatic status (aRel: HR=0.34 [95%CI 0.18,0.64]; sRel: HR=0.36 [95%CI 0.18,0.71]; LRT p=0.697) on PFS2 showed non-significant trends towards benefit. In particular, older patients appearing to derive less benefit from ASCT2 in terms of PFS2.
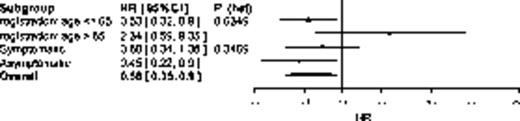
When OS by randomized treatment is considered in relation to age (²65 yrs: HR=0.53 [95%CI 0.32,0.90]; >65 yrs: HR=2.34 [95%CI 0.59,9.35]; LRT p=0.635) & symptomatic status (aRel: HR=0.44 [95%CI 0.22,0.90]; sRel: HR=0.68 [95%CI 0.34,1.36]; LRT p=0.347) non-significant trends towards further benefit can be observed (Fig 1b), reflected by the 4-year survival (ASCT2 - aRel: 75.5% vs sRel: 60.2%; logrank p=0.080 & NTC - aRel: 55.1% vs sRel: 49.0; logrank p=0.707) & age at trial entry (ASCT2 - ²65 yrs: 71.2% vs >65 yrs: 58.9; logrank p=0.726 & NTC - ²65 yrs: 47.9% vs >65 yrs: 68.2; logrank p=0.653).
Conclusion: These results show both a clear OS advantage to ASCT2 post bortezomib based re-induction therapy & that this advantage may well be improved in younger patients with biochemical, rather than symptomatic relapse. This data is key for patient-centered clinical decision-making & adds to previous analysis demonstrating a clear advantage to ASCT2 in terms of TTP and PFS in patients with MM at first relapse1.
1. G Cook, et al. The Lancet Oncology, Vol. 15, No. 8, p874-885.
Forest plot showing impact of age & symptomatic status at retreatment on (a) TTP and (b) OS of randomised treatment. To the left favors ASCT2 and to the right NTC.
(a) TTP
(b) OS
Forest plot showing impact of age & symptomatic status at retreatment on (a) TTP and (b) OS of randomised treatment. To the left favors ASCT2 and to the right NTC.
(a) TTP
(b) OS
Ashcroft:janssen: Consultancy, Research Funding; celgene: Consultancy, Honoraria; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Williams:Janssen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau. Cavenagh:janssen: Consultancy, Speakers Bureau; novartis: Consultancy, Speakers Bureau; celgene: Consultancy, Speakers Bureau; amgen: Consultancy, Speakers Bureau. Snowden:Sanofi: Consultancy; MSD: Consultancy, Other: Educational support, Speakers Bureau; Janssen: Other: Educational support, Speakers Bureau; Celgene: Other: Educational support, Speakers Bureau. Parrish:Celgene: Speakers Bureau; Janssen: Speakers Bureau. Yong:Janssen: Honoraria; BMS: Honoraria; Takeda: Honoraria; Novartis: Consultancy; Autolous: Consultancy; Amgen: Honoraria. Cavet:Celgene: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau. Bird:Pfizer: Consultancy; Novartis: Consultancy; Amgen: Consultancy; Celgene: Speakers Bureau; Janssen: Other: Educational support. Heartin:Janssen: Consultancy; Celgene: Speakers Bureau. O'Connor:Celgene: Research Funding. Brown:Janssen: Research Funding; Celgene: Research Funding; Roche: Research Funding; Bayer: Research Funding. Morris:Celgene: Other: Meeting support; Janssen: Other: Meeting support. Cook:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Chugai: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Jazz Pharma: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal