Abstract
Background: Allogeneic HSCT is a widely used treatment for children with acute leukemia (AL) either relapsed or at high risk of treatment failure. However, an HLA-identical sibling is available for only 20-25% of patients and an UD can be located in a suitable time only for a portion of the remaining population. HSCT from an HLA-haploidentical relative (haplo-HSCT) is now considered an alternative option, especially in view of the recent insights in graft manipulation. We recently developed a novel method of more selective T-cell depletion based on physical elimination of α/β T cells (ClinicalTrial.gov identifier: NCT01810120), shown to be effective for both preventing graft-versus-host disease (GvHD) and for conferring improved protection against infections in comparison to haplo-HSCT performed through the infusion of positively selected CD34+ cells. The initial results on 40 patients with AL were reported at the ASH Meeting in 2013 (Bertaina et al). We now present the comparison of the outcome of 80 children with AL given haplo-HSCT after α/β T-cell depletion (group 1) with that of patients transplanted from an HLA-identical sibling (group 2) or an UD (group 3) in the same time period.
Patients and methods: All patients with AL were transplanted at the Bambino Gesù Children's Hospital in Rome, Italy, between December 2010 and September 2014; 80 patients were included in group 1, 41 in group 2 and 51 in group 3. Patients were offered α/β T-cell-depleted haplo-HSCT in the absence of suitable conventional donor (HLA identical sibling or 10/10 UD evaluated using high resolution typing) or if affected by rapidly progressive disease not permitting time to identify an UD. Clinical characteristics of patients assigned to the 3 groups and those of their donor are shown in Table1. All children were given a fully myeloablative regimen. No group 1 patient was given any post-transplantation GvHD prophylaxis, while patients of group 2 and 3 were given Cyclosporine-A and short-term methotrexate. Group 1 and 3 patients received ATG Fresenius® (4 mg/Kg/day) from day -5 to -3 for preventing both graft rejection and GvHD.
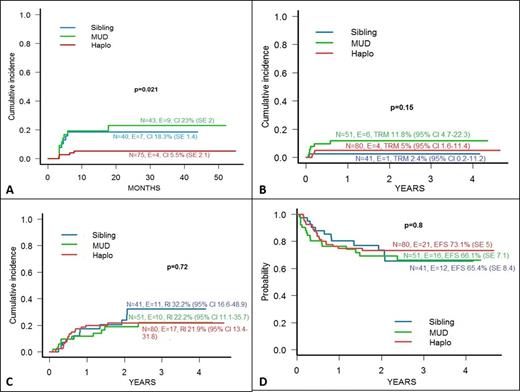
Results: All group 2 and 3 patients had sustained engraftment of donor cells, while 1 of the 80 patients included in group 1 experienced primary graft failure and was rescued by haplo-HSCT from the other parent. The cumulative incidence (CI) of acute GvHD was 30%, 41% and 42%, respectively. Remarkably, all children of the group 1 who developed acute GvHD had a skin-only involvement, while 17% and 16.3% of those of group 2 and 3 had either gut or liver involvement (p<0.001). The CI of chronic GvHD was significantly lower in group 1 children than in those of groups 2 and 3 (p=0.02, see also Figure 1-Panel A). None of the 4 group 1 patients experiencing chronic GvHD had the extensive form of the disease, while the CI of extensive chronic GvHD of group 2 and 3 was 8% and 14%, respectively (p=0.01). Four, 1 and 6 children of patients assigned in group 1, 2 and 3, respectively, died for transplant-related causes; the CI of transplantation-related mortality (TRM) in the 3 groups is shown in Figure 1-Panel B. Relapse was the main cause of treatment failure and occurred at a comparable CI in all the 3 groups (see also Panel C of Figure 1). The 3-year probability of Event-Free Survival (EFS) was comparable in the 3 groups (Figure 1 - Panel D). In multivariate analysis, a Total Body Irradiation (TBI)-containing regimen was the only variable favourably influencing EFS of group 1 children (hazard ratio 2.93, 95% Confidence Interval 1.24-6.95). No variable influenced EFS of group 2 and 3 patients.
Conclusions: Overall, these data indicate that haplo-HSCT after α/β T-cell depletion is associated with a risk of TRM and leukemia recurrence comparable to that of transplantation from an HLA-identical sibling or an UD, this translating in a similar probability of EFS. In view of the low incidence of chronic GvHD, this transplant option has to be considered a competitive alternative for children with AL in need of an allograft.
| . | Sibling (n=41) . | MUD (n=51) . | Haplo (n=80) . | . |
|---|---|---|---|---|
| Sex | p=0.77 | |||
| M | 27 | 32 | 55 | |
| F | 14 | 19 | 25 | |
| Age at Transplantation (years) | 10.6 | 9.4 | 9.7 | p=0.20 |
| Disease | p=0.23 | |||
| ALL | 34 | 35 | 56 | |
| AML | 7 | 16 | 24 | |
| Disease status at Transplantation | p=0.13 | |||
| CR1 | 20 | 30 | 30 | |
| CR2 | 21 | 20 | 47 | |
| ≥CR3 | 0 | 1 | 3 | |
| CMV serology (Donor/Recipient) | p=0.001 | |||
| neg/neg | 8 | 5 | 6 | |
| neg/pos | 8 | 21 | 7 | |
| pos/neg | 1 | 4 | 11 | |
| pos/pos | 24 | 21 | 56 | |
| Source of Stem Cells | p<0.0001 | |||
| BM | 40 | 40 | 0 | |
| PBSC | 1 | 11 | 80 | |
| Conditioning regimens | p=0.10 | |||
| TBI-based | 26 | 29 | 60 | |
| non TBI-based | 15 | 22 | 20 |
| . | Sibling (n=41) . | MUD (n=51) . | Haplo (n=80) . | . |
|---|---|---|---|---|
| Sex | p=0.77 | |||
| M | 27 | 32 | 55 | |
| F | 14 | 19 | 25 | |
| Age at Transplantation (years) | 10.6 | 9.4 | 9.7 | p=0.20 |
| Disease | p=0.23 | |||
| ALL | 34 | 35 | 56 | |
| AML | 7 | 16 | 24 | |
| Disease status at Transplantation | p=0.13 | |||
| CR1 | 20 | 30 | 30 | |
| CR2 | 21 | 20 | 47 | |
| ≥CR3 | 0 | 1 | 3 | |
| CMV serology (Donor/Recipient) | p=0.001 | |||
| neg/neg | 8 | 5 | 6 | |
| neg/pos | 8 | 21 | 7 | |
| pos/neg | 1 | 4 | 11 | |
| pos/pos | 24 | 21 | 56 | |
| Source of Stem Cells | p<0.0001 | |||
| BM | 40 | 40 | 0 | |
| PBSC | 1 | 11 | 80 | |
| Conditioning regimens | p=0.10 | |||
| TBI-based | 26 | 29 | 60 | |
| non TBI-based | 15 | 22 | 20 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal