Abstract
Background: We recently completed a prospective study (ClinicalTrial.gov identifier: NCT01810120) which showed that haplo-HSCT after depletion of α/β T cells is an effective option for those children in need of an allograft and lacking an immediately available HLA-identical related or unrelated donor. However, recovery of adaptive T-cell immunity remains suboptimal and some patients died due to viral infections in the early post-transplant period. Thus, strategies aimed at accelerating early recovery of adaptive T-cell immunity are desirable.
Study design and patients: We designed a phase I/II trial aimed at testing the safety and the efficacy of post-transplant infusion of donor-derived T cells transduced with the new iC9 suicide gene (BPX-501) in children with malignant or non-malignant disorders (ClinicalTrials.gov identifier: NCT02065869); enrollment started in December 2014. Cells are administered within 14 + 4 days after haplo-HSCT. The phase I portion of the trial consists of a classical 3+3 design with 3 cohorts, receiving escalating doses of BPX-501 cells of 2.5 x 105, 5 x105, and 1x106 cells/kg, respectively. Patients included in the phase II portion received the highest dose identified during the phase I portion of the study for a maximum of 60 children in both phase I/II portions of the study. As of July 25th 2015, 25 children have been screened and included in the study: 23 have been infused with BPX-501 cells. The analysis refers to the 16 patients with a minimum follow-up of 90 days after transplantation; they had acute lymphoblastic leukemia (ALL, 6), acute myeloid leukemia (1), severe combined immune-deficiency (4), Wiskott-Aldrich syndrome (3) and Fanconi Anemia (2). All children with acute leukemia were transplanted in morphological complete remission (CR). Median age at haplo-HSCT was 3.5 years (range, 03-17.8); 7 patients (44%) were females. All children received >10x106 CD34+ cells/Kg and <1x105 αβ+ T cells/Kg. There was no difference in graft composition between these 16 patients and those who were previously included in the study on haplo-HSCT after depletion of α/β T cells (historical controls).
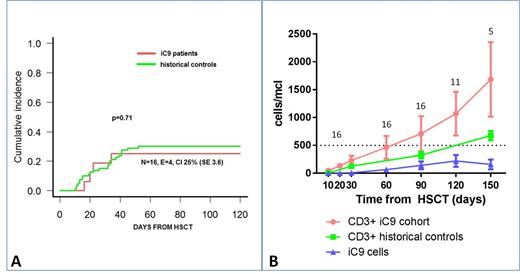
Results: BPX-501 cells were infused at a median time of 16 days (range 13-18); median cell viability post-thaw was 91% (range 65-97). Treatment was well tolerated and no infusion-related side effects were recorded. The recommended dose identified during the phase I of the trial to be used for the phase II portion was 1x106 cells/kg. Four children developed grade I-II skin only acute graft-versus-host disease (GvHD) at 16, 20, 22 and 34 days after haplo-HSCT, respectively, which resolved with topical steroids; no patient had either gut or liver acute GvHD. The 100-day cumulative incidence (CI) of skin-only grade I-II acute GvHD was 25% (SE 3.6); it was 30% (SE 2.1) in the historical controls (Figure 1 - Panel A). No patient developed chronic GvHD. In 4 patients, mixed chimerism present at time of BPX-501 cell infusion completely reverted to full donor chimerism. None of the 16 patients included in the analysis had graft failure or died of transplant-related complications. Two patients, both with ALL transplanted in CR3, relapsed at 86 and 153 days after the allograft, respectively. Median time to discharge after haplo-HSCT was 28 days (range, 19-86) as compared to 38 days (range, 18-174) in the historical controls (p=0.08). Four patients were re-hospitalized due to: cytomegalovirus (CMV) infection (2), fever of unknown origin (1) and valganciclovir-induced neutropenia (1). BPX-501 cells progressively expanded over time and are still persisting, potentially contributing to the recovery of adaptive T-cell immunity. The mean number of both CD3+ and BPX-501 cells at the different time-points are reported in Figure 1 - Panel B, which also details the data of historical controls.
Conclusions: Overall, these data indicate that the infusion of BPX-501 cells is safe and well tolerated. The 100-day CI of skin-only grade I-II acute GvHD observed in these patients is similar to that of children included in the previous trial of haplo-HSCT after depletion of α/β T cells. BPX-501 cells expand in vivo and persist over time, potentially contributing to accelerate the recovery of adaptive T-cell immunity, with improved clinical outcome. The iC9 cell-suicide system may increase the implementation of cellular therapy approaches aimed at optimizing immune recovery after transplantation.
Qasim:Cell Medica: Research Funding; Autolus Ltd: Consultancy, Equity Ownership, Research Funding; Miltenyi Biotec GmbH: Research Funding; Cellectis: Research Funding. Moseley:Bellicum Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal