Abstract
Introduction :
Acute myeloid leukemia (AML) is a heterogeneous disease with various cytogenetics and molecular abnormalities that lead to different clinical outcomes. The overall prognostic significance of specific mutations at diagnosis has been described. Some are associated with better prognosis (eg, mutations in the myeloid transcription factor gene CEBPA, mutations in the NPM1 gene in the absence of FLT3-ITD, downregulated HOX expression) and some with an adverse prognosis (eg, FLT3-ITD, high expression of the BAALC gene, overexpression of the ETS-related gene ERG, certain mutations in IDH1 and IDH2). However the impact of these mutations in a salvage setting is not well described.
Methods:
We retrospectively analyzed the patients with AML who received 1st or 2nd salvage treatment (Salvage 1 & Salvage 2) from September 2012 to June of 2015. All patients included in the study had received induction treatment at MD Anderson. The somatic mutations used in the study were obtained at the time of diagnosis. A total of 108 patients with known mutations who eventually received Salvage 1 were included; these also included the 41 patients who later received Salvage 2. Eight mutations (FLT3, CEBPA, IDH1, IDH2, TP53, NPM1, RAS, and JAK2) were evaluated for their correlation with outcome after salvage therapy, specifically event-free survival (EFS) and overall survival (OS) after salvage 1 and salvage 2. Any new evolving mutation after the treatment was also investigated.
Results:
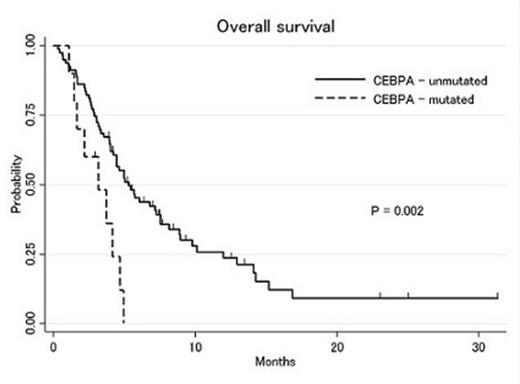
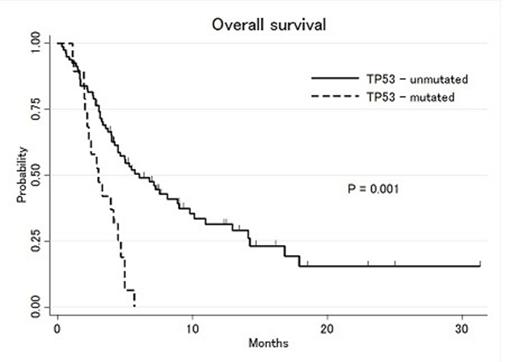
Out of 108 patients that received salvage 1 treatment, 27% patients had IDH mutations, 19% had TP53 mutations, 16% had RAS mutations, 15% had FLT3-ITD mutations, 11% had NPM1 mutations, 11% had CEBPA mutations and 6% had JAK2 mutations at the time of diagnosis. Median age was 65 years (range: 19-84), median WBC count 5.9 x 109/L (range: 0.8-250), bone marrow blast 45% (range: 0-93) and peripheral blood blast 15.5% (range: 0-96). Cytogenetic abnormalities according to SWOG/ECOG classification were 5% favorable, 50% intermediate, 41% unfavorable and 12 % indeterminate cytogenetics. Forty-two percent of patients were refractory to induction treatment and 58% had achieved complete remission and relapsed after a median of 7 months (range: 1-22). Complete remission and complete remission with incomplete count recovery (CR/CRi) rate after 1st salvage therapy was 30% (95% CI: 21-39%). Median EFS and OS were 2.2 and 5.0 months, respectively. Among 30 pts with repeat molecular analysis prior to salvage 1, some patients developed new mutations, including 4 that acquired FLT3-ITD, and 1 patient each with new TP53, NPM1 and RAS mutations. Using cox univariate analysis stratified by the cytogenetic risk group, CEBPA (HR: 2.3, 95%CI: 1.1-4.7), TP53 (HR: 2.3, 95%CI: 1.1-4.7), JAK2 (HR: 3.3, 95%CI: 1.3-7.9) mutations were significantly associated with shorter OS after start of salvage 1 (Figure 1&2). Patients with CEBPA and/or TP53 and/or JAK2 mutations had a significantly shorter median OS compared to patients without any of these mutations (2.9 vs 7.6 months, respectively). IDH1, IDH2, FLT3-ITD, NPM1 and RAS mutations were not significantly associated with survival outcome.
Among these 108 patients, 41 (38%) later received salvage 2 therapy. After Salvage 1 1 of 5 pts assessed developed a new mutation (TP53) prior to salvage 2. Overall remission (CR/CRi) rate was 29% (95%CI: 16-46%) with 2nd salvage therapy. Median OS was 3.5 months and EFS 1.5 months. Using Cox-univariate analysis stratified by the cytogenetic risk, CEBPA mutation (HR: 8.7, 95%CI: 2.3-33.0) and TP53 mutation (HR: 5.9, 95%CI: 1.3-26.2) were significantly associated with shorter OS.
Conclusion:
This analysis suggests that CEBPA and TP53 mutations are associated with shorter OS in patients receiving 1st or 2nd salvage therapy after failure of induction therapy. The adverse prognostic influence of CEBPA is particularly notable and requires further study. In addition, occasionally new mutations may arise after treatment failure. Mutation profiling at diagnosis as well as prior to each salvage treatment can thus be used for risk stratification of patients undergoing salvage treatment and to consider for targeted drugs to improve the outcome.
Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding. Cortes:Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Teva: Research Funding; Pfizer: Consultancy, Research Funding; BerGenBio AS: Research Funding; Ariad: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Ambit: Consultancy, Research Funding; Arog: Research Funding; Celator: Research Funding; Jenssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal