Abstract
Posttransplant lymphoproliferative disorder (PTLD) is a potentially fatal disorder arising after solid organ transplant (SOT) or hematopoietic stem cell transplant (HSCT). Iatrogenically impaired immune surveillance and Epstein-Barr virus (EBV) primary infection/reactivation are key factors in the pathogenesis. However, current knowledge on all aspects of PTLD is limited due to its rarity, morphologic heterogeneity, and the lack of prospective trials. Furthermore, the broad spectrum of underlying immune disorders and the type of graft represent important confounding factors. Despite these limitations, several reviews have been written aimed at offering a guide for pathologists and clinicians in diagnosing and treating PTLD. Rather than providing another classical review on PTLD, this “How I Treat” article, based on 2 case reports, focuses on specific challenges, different perspectives, and novel insights regarding the pathogenesis, diagnosis, and treatment of PTLD. These challenges include the wide variety of PTLD presentation (making treatment optimization difficult), the impact of EBV on pathogenesis and clinical behavior, and the controversial treatment of Burkitt lymphoma (BL)-PTLD.
Case reports
Case 1
A 19-year-old woman with end-stage renal failure due to congenital kidney disease underwent a living-donor renal transplant in 2006. Eight years later, she was diagnosed with EBV+MYC translocation–confirmed BL-PTLD stage IVB (Figure 1) while on treatment with mycophenolate mofetil (MMF), tacrolimus, and low-dose steroids. EBV viral load was very high (5.84 log IU/mL). Due to the aggressive presentation, immune suppression was completely discontinued and immunochemotherapy with rituximab and CHOP (cyclophosphamide-doxorubicin-vincristine-prednisone) was started. Partial metabolic response was obtained after 4 cycles of chemotherapy (Figure 1). However, at the start of the sixth cycle, the patient complained of headache; clinical examination showed a peripheral facial nerve paresis. Morphologic examination of the cerebrospinal fluid confirmed the presence of a BL relapse with high EBV viral load, whereas brain magnetic resonance imaging did not reveal cerebral or cerebellar involvement. After treatment with intrathecal methotrexate, clinical symptoms disappeared, with normalization of the cerebrospinal fluid. Consolidation therapy with 4 cycles of high-dose methotrexate (3.5 g/m2) was given. A final evaluation with 18F-FDG–PET/CT, bone marrow examination, and brain magnetic resonance imaging showed complete remission. Lumbar puncture, however, showed the presence of meningeal disease, and rescue treatment with intrathecal rituximab and systemic etoposide and cytarabine was initiated, leading to an ongoing complete remission.
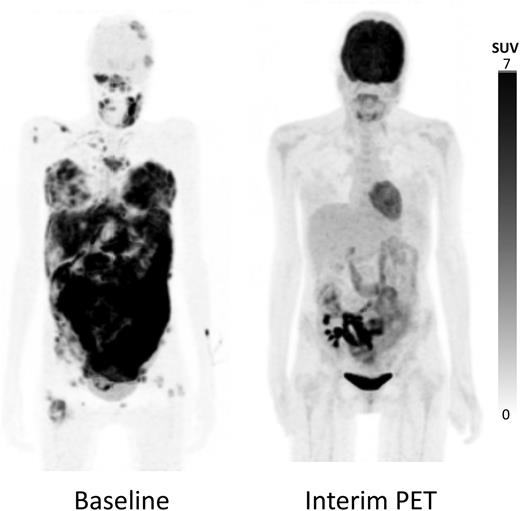
Maximum-intensity projection 18F-FDG–PET/CT images. Baseline image showed multiple supra- and infradiaphragmatic nodal lesions and extranodal lesions in breast, intestines, and bone marrow (left); 18F-FDG–PET/CT after 4 cycles of therapy showed a complete metabolic response in all nodal and extranodal lesions, with the exception of limited residual hypermetabolic lesions in the intestinal tract adjacent to the kidney transplant in the right iliac fossa (right). 18F, radionuclide fluorine 18; FDG, fluorodeoxyglucose; PET, positron emission tomography; CT, computed tomography.
Maximum-intensity projection 18F-FDG–PET/CT images. Baseline image showed multiple supra- and infradiaphragmatic nodal lesions and extranodal lesions in breast, intestines, and bone marrow (left); 18F-FDG–PET/CT after 4 cycles of therapy showed a complete metabolic response in all nodal and extranodal lesions, with the exception of limited residual hypermetabolic lesions in the intestinal tract adjacent to the kidney transplant in the right iliac fossa (right). 18F, radionuclide fluorine 18; FDG, fluorodeoxyglucose; PET, positron emission tomography; CT, computed tomography.
Case 2
A 36-year-old woman with end-stage renal failure due to hemolytic uremic syndrome underwent a deceased-donor renal transplant in 1981 and in 1999. Twelve years after the second transplant, she was referred to our center for investigations because of abdominal pain. 18F-FDG–PET/CT revealed a large intense hypermetabolic nonobstructive cecal and terminal ileal wall thickening, with mesenteric and retroperitoneal lymphadenopathies and diffuse omental, mesenteric, and peritoneal involvement in combination with supradiaphragmatic lymphadenopathies (stage IVA). Biopsy of the mass revealed an EBV− diffuse large B-cell lymphoma (DLBCL). EBV viral load in peripheral blood was not detectable. At that moment, immune suppressive therapy consisted of MMF, tacrolimus, and low-dose steroids. Reduction of immune suppression (RIS; cessation of MMF and dose reduction of tacrolimus) in combination with rituximab was initiated. However, progressive disease was rapidly observed, and immunochemotherapy with rituximab and CHOP was started, leading to a complete remission. Three months later, the patient relapsed, necessitating treatment with intensive chemotherapy (cytarabine-cisplatin-dexamethasone), followed by high-dose chemotherapy and autologous HSCT. Three years after HSCT, there is no evidence of disease recurrence, and renal function is stable under immunosuppressive treatment with low-dose steroids and everolimus, a mammalian target of rapamycin inhibitor.
Diagnostic challenges
PTLD comprises a wide spectrum of lymphoid and plasmacytic proliferations occurring after SOT or allogeneic HSCT.1 This spectrum of morphologic appearances varies in terms of cellular constituents, degree of resemblance to reactive or neoplastic lesions known in immunocompetent hosts, and association with the herpesvirus EBV. Prior to diagnosing PTLD, it is important to exclude specific and nonspecific lymphoplasmacytic infiltrations associated with infection, graft rejection, graft-versus-host disease, or recurrence from a known lymphoma that developed prior to transplant.
After it is histologically proved, PTLD is categorized as precisely as possible by using the 4 categories provided by the current 2008 World Health Organization (WHO) classification (Figure 2)2 :
Early-type PTLDs are nondestructive lymphoplasmacytic proliferations that are further subdivided in plasmacytic hyperplasia and infectious mononucleosis–like paracortical hyperplasia. Some authors include florid follicular hyperplasia in this category, but lymphadenopathy characterized by reactive nodular paracortical dermatopathic changes or sinus histiocytosis is generally not considered PTLD.3
Polymorphic PTLDs are the most challenging presentations because they are destructive lymphoplasmacytic proliferations that do not fulfill the strict criteria of a malignant lymphoma. In certain cases, this subtype can be problematic to differentiate from prominent infectious mononucleosis–like lesions and often show Hodgkin lymphoma (HL)-like features.4 In polymorphic cases, the infiltrate comprises a minority of transformed B-blasts in a polymorphic background of lymphocytes, histiocytes, and plasma cells. These B-blasts show a spectrum of morphologic features varying from activated immunoblasts to Hodgkin cells and to fully developed Reed-Sternberg cells. They strongly express CD20 and CD30 and generally lack CD15.
Monomorphic PTLDs should be the most straightforward to diagnose because they fulfill the histopathological criteria of a lymphoma recognized in immunocompetent hosts. The majority is of B-cell phenotype (with DLBCL, BL, and plasmablastic lymphoma as most prevalent subtypes), but T-cell lymphomas (like hepatosplenic T-cell lymphoma) and even some composite lymphomas have been described.5-9 Indolent B-cell lymphomas are, at present, not considered PTLDs, even when occurring in transplant patients.
Sporadically, classical HL is also diagnosed in the context of posttransplantation when typical HRS cells are present in the appropriate cellular background of plasma cells, eosinophils, and (epithelioid) histiocytes. The HRS cells show strong reactivity for CD30 and CD15, along with absent CD20 and weak PAX5 expression.10
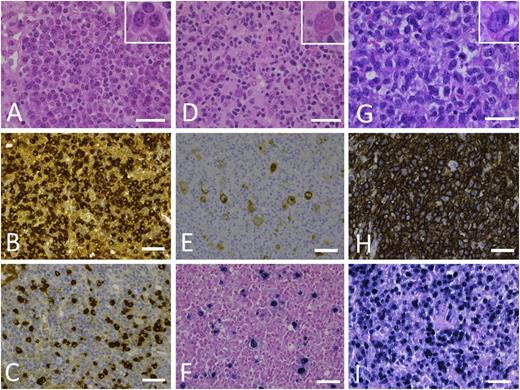
Morphologic spectrum of PTLD. (A-C) PTLD, early lesion, plasmacytic hyperplasia. (A) In a preserved underlying architecture, there is a proliferation of small reactive plasma cells (inset). Immunohistochemical staining against κ (B) and λ (C) light chains shows the polytypic character of the plasma cells. (D-F) PTLD, polymorphic. (D) Lymph node architecture is effaced by a polymorphic proliferation of B cells of variable size, shape, and degree of transformation (HRS-like cell shown in inset), which are admixed with numerous small lymphocytes, plasma cells, eosinophils, and histiocytes. (E) Immunohistochemical staining against CD20 shows membranous and often Golgi-type expression in the B blasts. (F) Epstein-Barr virus–encoded RNA in situ hybridization shows nuclear positivity not only in the HRS-like cells but also in a variety of B cells. (G-I) PTLD, monomorphic, EBV+ DLBCL. (G) Monotonous proliferation of large transformed B cells (inset) with an infiltrative growth pattern and mitotic activity. (H) Diffuse CD20 expression of the infiltrate corresponding to the B-cell phenotype. (I) Most cells are EBV+, as seen in this Epstein-Barr virus–encoded RNA in situ hybridization. Images were taken with a Leica DFC290. Bars represent 50 µm. Original magnification 630×. Hematoxylin and eosin stain. HRS, Hodgkin/Reed-Sternberg.
Morphologic spectrum of PTLD. (A-C) PTLD, early lesion, plasmacytic hyperplasia. (A) In a preserved underlying architecture, there is a proliferation of small reactive plasma cells (inset). Immunohistochemical staining against κ (B) and λ (C) light chains shows the polytypic character of the plasma cells. (D-F) PTLD, polymorphic. (D) Lymph node architecture is effaced by a polymorphic proliferation of B cells of variable size, shape, and degree of transformation (HRS-like cell shown in inset), which are admixed with numerous small lymphocytes, plasma cells, eosinophils, and histiocytes. (E) Immunohistochemical staining against CD20 shows membranous and often Golgi-type expression in the B blasts. (F) Epstein-Barr virus–encoded RNA in situ hybridization shows nuclear positivity not only in the HRS-like cells but also in a variety of B cells. (G-I) PTLD, monomorphic, EBV+ DLBCL. (G) Monotonous proliferation of large transformed B cells (inset) with an infiltrative growth pattern and mitotic activity. (H) Diffuse CD20 expression of the infiltrate corresponding to the B-cell phenotype. (I) Most cells are EBV+, as seen in this Epstein-Barr virus–encoded RNA in situ hybridization. Images were taken with a Leica DFC290. Bars represent 50 µm. Original magnification 630×. Hematoxylin and eosin stain. HRS, Hodgkin/Reed-Sternberg.
It should be noted that exact classification in 1 of these 4 categories will not always be possible because of overlap between several categories or because PTLDs can present as different morphologic subtypes within different locations in the body or even within a single biopsy sample. The latter finding is in concordance with the hypothesis that in some cases, there might be a progressive transition from early PTLD through polymorphic PTLD to monomorphic PTLD.11 It might therefore be useful to consider performing a new biopsy when PET imaging suggests a more malignant disease presentation than observed in the biopsy sample (especially after core needle biopsy).12 Factors causing this presumed transition are not defined, and this evolution is clearly not present in all cases, as illustrated by the observation of extremely aggressive monomorphic PTLD cases occurring <2 weeks post-HSCT.
The HL-like polymorphic PTLD subtype is among the most difficult to diagnose because there is a clear overlap with classical HL.13,14 Correlation with the clinical presentation is important, because classical HL will not primarily involve mucosa-associated lymphoid tissues, whereas HL-PTLDs generally do. Differentiating HL-like polymorphic PTLD from HL-PTLD is important, given the often aggressive presentation and poor prognosis in the former group of patients.4 However, this worse prognosis is not a constant finding, as illustrated by EBV+ mucocutaneous ulcers.15,16
Other diagnostic challenges include cases with more extensive plasmacytic infiltrates or plasmacytoma-like lymphoproliferations and borderline cases that fall between polymorphic and monomorphic PTLD. It is generally assumed that the biological behavior will depend on the most malignant subtype, but well-defined criteria are lacking.
Clonality studies can support the diagnosis because monomorphic PTLDs typically show clonal immunoglobulin or T-cell receptor gene rearrangements in the B-cell or T-cell populations, respectively. Of interest, due to the immunosuppressed state, monomorphic B-cell PTLDs often contain (oligo)clonal reactive restricted T-cell populations that can be detected on T-cell receptor polymerase chain reaction (PCR).17 These T-cell populations, however, should not be considered composite lymphomas unless they fulfill the histopathological criteria for T-cell lymphoma. Polyclonal B- and T-cells compose early lesions, whereas clonal B-cell populations can be detected in polymorphic PTLDs, typically on a polyclonal background.
It should be noted that EBV positivity is not a prerequisite for the diagnosis of PTLD, and the number of EBV− cases increased over time from 10% (1990-1995) to 48% (2008-2013).18 It is currently under debate whether EBV− PTLDs are to be considered immunosuppression related or coincidental, because some molecular genetic studies suggest similar pathogenic mechanisms for EBV− PTLDs and EBV− lymphomas in immunocompetent hosts, but different from EBV+ PTLDs, justifying a different therapeutic approach for EBV− and EBV+ PTLDs.19-25 It is therefore recommended to determine EBV association in every biopsy sample, using Epstein-Barr virus–encoded RNA in situ hybridization as the gold standard.26 In addition, immunohistochemistry for viral proteins (eg, latent membrane proteins and EBV nuclear antigens) can provide information on the latency or lytic stage of the virus (as evidenced by ZEBRA protein expression). If EBV is detected in early, polymorphic, and monomorphic PTLDs, it is characterized by a broad viral latency (type III), sometimes in the presence of some lytic reactivation.27,28 In contrast to classical HL, in which EBV is typically restricted to the fully developed HRS cells and of intermediate latency (type II), EBV is present in a wide spectrum of B-cells in the HL-like polymorphic PTLD.13,14
Associations with other viruses, such as human herpesvirus 8 or cytomegalovirus, have been described in case reports, the former mainly coexisting with EBV and the latter being regarded as an epiphenomenon rather than a driver of the disease.29-31
Impact of transplanted organ type on PTLD: does it matter?
In addition to pretransplant EBV mismatch (the most important risk factor for developing PTLD) and the immune suppressive agents used to prevent rejection, the type of transplant organ also determines the risk for PTLD. Based on large transplant registries and smaller single-center retrospective analyses, the incidence of PTLD seems to be highest in haploidentical HSCT, heart/lung, and multivisceral transplants (up to 20%), followed by liver (4.5%), combination heart-lung (2.5%), pancreas (2%), kidney (1%-1.5%), and matched related and unrelated HSCT (0.5%-1%).32,33 Apart from histoincompatibility, the amount of lymphoid tissue present in the organ can partially explain the differences in PTLD development associated with different grafts. Grafts containing a substantial amount of lymphoid tissue (eg, small intestine) result in transfer of (potentially EBV-infected) donor lymphocytes, which may contribute to PTLD development.10
In contrast to SOT, PTLD after HSCT is almost exclusively of donor origin and develops during the first 6 months after transplant. This unique feature is a consequence of the profound T cell–depleting conditioning regimen, leading to lack of EBV-specific T cells and, hence, the often impressive and rapid growth of an EBV+ clone, even within the first weeks.34 Because immune reconstitution occurs in the first 6 to 12 months and oral immune suppression can often be stopped, late PTLD is rare after HSCT. However, an interesting finding is the increased incidence of late-onset HL in patients undergoing HSCT. In this population, a standardized incidence ratio of 6.2 was observed, whereas a standardized incidence ratio after SOT is ∼3.7.35,36 The lack of association with low CD4 counts is in line with the observation of typically normal CD4 counts in HIV+ patients diagnosed with HL.37
The incidence of PTLD has clearly increased during the last decade.18,33,38 This increase can be explained by several reasons, including the use of more potent immune suppression, the older age of both donor and recipient, the increased use of haploidentical HSCT, the increased awareness, and the prompt request for a biopsy in the case of PTLD suspicion. A notable exception seems to be the decreasing incidence in (at least pediatric) liver transplant recipients, possibly due to the use of minimal immune suppression strategies and to serial EBV monitoring.39
Whether the type of transplant organ predisposes to a specific lymphoma subtype is currently not clear. Although the numbers are limited and based on small series or case reports, plasmablastic lymphoma seems to be observed more frequently in heart transplant recipients compared with other transplant recipients.7,40 A similar observation has been reported for primary central nervous system (CNS) lymphoma, which is predominantly seen after kidney transplant (79% in a large multicenter international analysis).41
Staging and follow-up: what’s the evidence?
Accurate staging and assessment of response and end-of-treatment remission are important for optimal management of patients with lymphoma. Imaging plays a major role herein, and 18F-FDG–PET/CT has become the standard to assess pretreatment evaluation and therapy response in FDG-avid lymphomas.42,43 The superiority of 18F-FDG–PET/CT for end-of-treatment response assessment has also been extensively documented, with additional value seen mainly in patients with a radiologically unconfirmed complete response or partial response.44,45
Because 18F-FDG–PET/CT is superior to CT alone in evaluating nodal and extranodal involvement, and due to the similarity between PTLD and non-HL[NHL]/HL, 18F-FDG–PET/CT is routinely used in the diagnostic workup of PTLD patients. Several single-center studies have demonstrated a high sensitivity and specificity for detecting both nodal and extranodal involvement.12,46,47
To date, reports on the value of 18F-FDG–PET/CT to assess treatment response are very scarce, but several case reports have demonstrated the potential of this technique to evaluate treatment response.48-50 The recommendations for 18F-FDG–PET/CT to assess treatment response can be extrapolated to PTLD patients because of the resemblance with other FDG-avid NHLs; 18F-FGD–PET/CT is currently the standard of care at end of treatment.
There are currently no data available on the role of 18F-FDG–PET/CT for routine surveillance in PTLD patients, but similar to the recommendations for HL and FDG-avid NHL, the use of 18F-FDG–PET/CT in follow-up should be limited to patients with a clinical suspicion for recurrent disease.
Toward a uniform treatment of PTLD: the curious case of BL
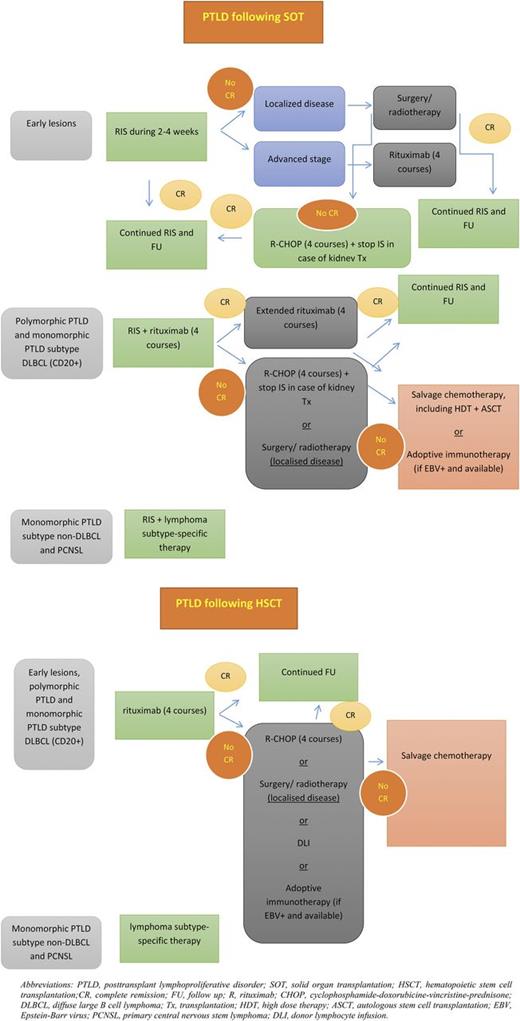
Because the development of PTLD is the consequence of an imbalance between immunosuppression and immunosurveillance, different approaches can be justified for treating this disorder, including RIS, destruction of the malignant clone, and suppression of the (EBV) viral load.51 Based on several phase 2 trials, rituximab (after RIS) is now considered standard therapy for most CD20+ PTLDs, including polymorphic and monomorphic DLBCL subtypes.52-57 For CD20− monomorphic PTLDs (including plasmablastic, plasma cell myeloma/plasmacytoma-like, and T-cell lymphoma) and for primary CNS lymphomas, most clinicians agree to treat these patients according to their immune-competent counterparts. Our therapeutic approach and the main advantages and disadvantages of different treatment options are shown in Figure 3 and Table 1, respectively.
Proposed treatment algorithm for PTLD after SOT or HSCT. ASCT, autologous stem cell transplant; CR, complete remission; DLI, donor lymphocyte infusion; FU, follow-up; HDT, high-dose therapy; IS, immune suppression; PCNSL, primary CNS lymphoma; R-CHOP, sequential immunochemotherapy with rituximab followed by CHOP; Tx, transplant.
Proposed treatment algorithm for PTLD after SOT or HSCT. ASCT, autologous stem cell transplant; CR, complete remission; DLI, donor lymphocyte infusion; FU, follow-up; HDT, high-dose therapy; IS, immune suppression; PCNSL, primary CNS lymphoma; R-CHOP, sequential immunochemotherapy with rituximab followed by CHOP; Tx, transplant.
Treatment options in PTLD
| Treatment . | Target . | Advantages . | Disadvantages . |
|---|---|---|---|
| RIS | T-cell function | High response rates in early lesions Role in preemptive therapy | Takes time, so not feasible in very aggressive presentations Organ dependent Risk for organ rejection Less efficacy in HSCT |
| Cytokine therapy | T-cell function B-cell mass | Promising response rates | High toxicity (not further developed) |
| Donor lymphocyte infusion | T-cell function EBV | High response rates Rapidly available (at least in related donors) | Only in HSCT Unfavorable toxicity profile (graft-versus-host disease) |
| Adoptive immunotherapy (EBV-specific cytotoxic T cells) | T-cell function EBV | Promising results in refractory PTLD Excellent toxicity profile Rapidly developing field | Only in EBV+ cases Time consuming High costs Availability restrictions |
| Surgery and radiotherapy | B-cell mass | Rapid symptom relief | Only in limited-stage (stage I) disease Mostly palliative |
| Chemotherapy | B-cell mass | High response rates | High treatment-related morbidity and mortality |
| Rituximab | B-cell mass | High response rates Favorable toxicity profile Induces better performance state Allows risk stratification Role in preemptive therapy | Only in CD20+ PTLD Specific side effects (progressive multifocal leukoencephalopathy, hypogammaglobulinemia, hepatitis B reactivation) |
| Antiviral agents | EBV | Promising role in combination with viral thymidine kinase–inducing agents (eg, arginine butyrate) but not further developed | No efficacy in monotherapy (lack of viral thymidine kinase expression in EBV+ PTLD) Only in EBV+ cases |
| IV immunoglobulins | EBV | Theoretically attractive due to the presence of antibodies against EBV proteins | Mostly combined with other therapies; hence, no information on real efficacy |
| Treatment . | Target . | Advantages . | Disadvantages . |
|---|---|---|---|
| RIS | T-cell function | High response rates in early lesions Role in preemptive therapy | Takes time, so not feasible in very aggressive presentations Organ dependent Risk for organ rejection Less efficacy in HSCT |
| Cytokine therapy | T-cell function B-cell mass | Promising response rates | High toxicity (not further developed) |
| Donor lymphocyte infusion | T-cell function EBV | High response rates Rapidly available (at least in related donors) | Only in HSCT Unfavorable toxicity profile (graft-versus-host disease) |
| Adoptive immunotherapy (EBV-specific cytotoxic T cells) | T-cell function EBV | Promising results in refractory PTLD Excellent toxicity profile Rapidly developing field | Only in EBV+ cases Time consuming High costs Availability restrictions |
| Surgery and radiotherapy | B-cell mass | Rapid symptom relief | Only in limited-stage (stage I) disease Mostly palliative |
| Chemotherapy | B-cell mass | High response rates | High treatment-related morbidity and mortality |
| Rituximab | B-cell mass | High response rates Favorable toxicity profile Induces better performance state Allows risk stratification Role in preemptive therapy | Only in CD20+ PTLD Specific side effects (progressive multifocal leukoencephalopathy, hypogammaglobulinemia, hepatitis B reactivation) |
| Antiviral agents | EBV | Promising role in combination with viral thymidine kinase–inducing agents (eg, arginine butyrate) but not further developed | No efficacy in monotherapy (lack of viral thymidine kinase expression in EBV+ PTLD) Only in EBV+ cases |
| IV immunoglobulins | EBV | Theoretically attractive due to the presence of antibodies against EBV proteins | Mostly combined with other therapies; hence, no information on real efficacy |
In this discussion, we mainly focus on BL-PTLD, a special subtype of monomorphic CD20+ PTLD. Guidelines on optimal treatment of BL-PTLD are lacking for several reasons. First, BL-PTLD is a very rare disorder that has been studied less intensively compared to other BL subtypes (eg, HIV associated). Second, similarly to sporadic BL, BL-PTLD affects both pediatric and adult transplant patients, hindering registration and application of uniform treatment protocols. Lastly, some BL-PTLD cases are MYC negative and characterized by the 11q-gain/loss pattern.24 Thus, the frequent lack of cytogenetic/fluorescence in situ hybridization data in published series and case reports makes distinction between true BL and BL-like cases impossible. The main questions concerning BL-PTLD are (1) does the clinical behavior of this disorder mimic other BL subtypes and, consequently, (2) does the proposed management strategy in other subtypes (ie, intensive multiagent chemotherapy, rituximab, and, in the case of HIV-associated BL, reconstitution of the immune system) also apply to patients presenting with BL-PTLD or is less-intensive treatment (rituximab and low-dose chemotherapy in combination with RIS) more desirable? Therefore, we performed a literature search and identified 6 small case series (3 pediatric and 3 adult) focusing on treatment of aggressive PTLD, including 39 patients with BL-PTLD.58-63 Twenty-five of 26 evaluable cases (those with information on MYC) had a proven MYC rearrangement. In contrast to sporadic (30%) and HIV-associated BL (25%-40%), most cases (82%) were EBV associated. Of interest, 90% of the cases were late-onset PTLDs (>1 year after transplant). In the 3 pediatric case series, treatment ranged from RIS and low-dose immunochemotherapy (rituximab-cyclophosphamide-prednisone) to RIS and intensive BL regimens, all leading to similar long-term outcome (75%-100% long-term survivors).58-60 In 2 adult case series, patients were treated with RIS, followed by short intensive chemotherapeutic regimens.61,62 Although response rates were acceptable (75%), toxicity was high, with 3 toxic deaths in 5 patients in the series described by Xicoy et al.62 In a recent case series from the German Study Group on PTLD, 5 of 8 adult patients received sequential immunochemotherapy (4 courses of rituximab followed by 4 courses of CHOP). Four of 5 patients reached complete remission, of which 1 had an early relapse. No intrathecal prophylaxis was given. The investigators concluded that sequential immunochemotherapy was a safe and effective treatment of BL-PTLD.63
Taken together, these studies clearly indicate that no firm conclusions can be made regarding the optimal treatment of pediatric and adult patients presenting with BL-PTLD, a statement confirmed by the recent British guidelines on the management of PTLD.64 Similarly to the efforts made in HIV-related BL that have led to a promising trial with a low-intensity immunochemotherapeutic regimen, prospective trials are urgently needed because initial optimal therapy (including the need for intrathecal chemotherapy) is of extreme importance to avoid difficult-to-treat relapses often localized in the CNS, as illustrated in case 1.65 The dramatic evolution of this case has changed our institutional protocol for BL-PTLD from sequential immunochemotherapy with rituximab followed by CHOP to a more intensive regimen including CNS prophylaxis.
Is there a difference between EBV+ and EBV− PTLD?
Despite the strong association between EBV and PTLD, 33% to 48% of PTLD cases are EBV− (as in case 2).18,33,38,66 The etiology of these PTLD cases is currently unknown. A number of hypotheses, such as hit-and-run EBV infection, other infectious agents, and chronic immune triggering by the graft, have been proposed as possible pathogenic mechanisms of these EBV− PTLDs.29,67-72 However, there is limited evidence supporting these theories. From a clinical viewpoint, EBV− cases tend to occur later (years) compared to EBV+ PTLD (months), corresponding to the fact that positive Epstein-Barr virus–encoded RNA staining is a constant finding in early lesions and polymorphic PTLD, with both (especially the former) subtypes observed predominantly in the early posttransplant period. Gene expression profiling studies suggest EBV− cases should not be considered real PTLD but rather “classical” lymphomas coincidentally occurring in a transplant recipient.23 This hypothesis is supported by a genomic study demonstrating that patients with EBV− posttransplant DLBCL share several common imbalances with DLBCL in immunocompetent patients, whereas both groups significantly differentiate from EBV+ posttransplant DLBCL.25 However, the fact that some of these lymphomas respond well to RIS likely makes this hypothesis too simplified.73
We previously suggested that the composition of the microenvironment may represent an important difference between EBV+ and EBV− PTLD (Morscio et al23 and unpublished results, T.T. and D.D.), influencing lymphomagenesis. Several genes involved in immunotolerance were upregulated in EBV+ cases and potentially contribute to the early onset of EBV+ compared to EBV− PTLD. Numerous studies have described how the presence or absence of particular stromal cells may impact prognosis in DLBCL patients.74,75 However, we only begin to understand the multitude of interactions that take place. In the case of EBV+ lymphomas, these studies are complicated by the fact that in most lesions, a fraction of the tumor cells is negative for EBV-encoded RNA and proteins. The reason for this is unclear, but this observation raises a number of important questions. Does the microenvironment vary in the immediate surroundings of EBV+ and EBV− tumor cells? And what are the implications for EBV-targeted therapy?
Whether the prognosis of EBV− cases differs from their EBV+ counterparts is currently not clear, given the controversial results in the literature. In the recently published analysis of 70 patients included in the international multicenter prospective phase 2 PTLD-1 trial, EBV association was not found to be a significant factor for overall survival or time to progression.57 Thus, there is currently no evidence that upfront treatment of EBV− and EBV+ PTLD should be different; of course, this does not apply to the use of EBV-specific adoptive immunotherapy, which is restricted to EBV+ cases.
Can we use EBV viral load for diagnosis and prevention of PTLD?
Given the important role of EBV in the pathogenesis of a substantial proportion of cases, many authors have investigated the role of viral load, determined by quantitative PCR in the diagnosis of PTLD. Although there seems to be a clear link between EBV proliferation and the development of EBV+ PTLD, especially in high-risk transplants such as pediatric liver and bowel transplant and HSCT, EBV viral load cannot replace biopsy as the gold standard for diagnosing PTLD.76,77
Measurement of EBV viral load has, however, emerged as a useful tool to initiate preemptive treatment. This strategy, in which immune suppression is reduced and/or rituximab is administered guided by EBV viral load, has shown promising results in SOT and HSCT.78-80 Impressive results with very favorable toxicity profiles have also been obtained by prophylactic and preemptive administration of EBV-specific cytotoxic T lymphocytes (CTLs).81 Despite these results, wide applicability of this approach has been limited due to high costs, the labor-intensive procedure, and availability difficulties. However, new strategies to develop more rapid approaches for generating virus-specific T cells and the introduction of a third-party bank with HLA-typed EBV-specific CTLs might alter the therapeutic landscape of PTLD in the near future.82-85 In addition, recent studies in mice have shown that adoptive transfer of pamidronate-expanded Vγ9Vδ2 T cells and tacrolimus-resistant engineered CTLs might become potential therapies in PTLD without the need for reducing immune-suppressive therapy.86,87
To improve the positive predictive value of EBV viral load, different approaches have been described that combine EBV PCR with measurements of diminished T-cell immunity (absolute lymphocyte count, absence of EBV-specific CD8+ T cells) or cytokine polymorphisms.88-90 Other potential candidates allowing preventive or prophylactic interventions include measurements of interleukin-6, interleukin-10, and chemokine (C-X-C motif) ligand 13.91-93 However, these approaches have not yet been validated in larger series.
Prognostic factors in PTLD
Compared to DLBCL in immune-competent patients, prognosis of PTLD is poor, with observed 5-year overall survival rates ranging from 40% to 60%.33,38,66 Although most of these deaths are associated with progressive disease, up to 40% of patients will eventually die due to unrelated causes (mainly infections), emphasizing the fragility of transplant patients, even in complete remisson.33 During the last decade, several prognostic indices have been proposed by different authors, but validation of these scores in different transplant populations has shown conflicting results, mainly due to heterogeneity in design, patient population, and treatment.38,56,94-97 In most series, classical factors, including higher age, advanced disease, poor performance state, elevated lactate dehydrogenase, and CNS invasion, are also associated with poor prognosis in PTLD patients. Recently, hypoalbuminemia was also established as a very strong risk factor.38 Based on recent literature, we consider the International Prognostic Index (IPI)98 as a reliable and significant predictor of survival in patients with PTLD.33,99,100 In addition to demonstrating the prognostic role of the IPI score, a large prospective phase 2 trial evaluating the sequential use of rituximab and CHOP chemotherapy in patients with CD20+ PTLD after SOT showed that response to rituximab monotherapy predicted overall survival.57 This finding led to an amendment of the trial, introducing the concept of risk-stratified sequential treatment according to the response to rituximab. In contrast, thoracic organ transplant recipients not responding to rituximab monotherapy had a particularly poor prognosis and chemotherapy-refractory disease.100 Based on these findings, a new multicenter prospective trial (PTLD-2) has been initiated, in which risk groups are not only defined on the basis of response to rituximab monotherapy but also on the IPI and the type of transplanted organ (https://clinicaltrials.gov; NCT02042391).
Conclusion and future perspectives
The increasing number of publications on PTLD, the detailed description of this entity in the WHO 2008 classification, and the international cooperation of different clinical and research groups have all contributed to an increased knowledge on the different aspects of the disorder. However, as illustrated in Table 2, many questions and challenges remain, making further research and clinical studies mandatory in this rapidly changing field of ever-increasing transplant activities worldwide and the use of new and very potent immunosuppressive therapies.
Perspectives and future challenges in PTLD
| Aspect . | Research/clinical challenges/opportunities . |
|---|---|
| Incidence | Integrating large registries and complete multicenter/nationwide detailed information, preferentially prospective |
| Risk factors | Searching for tools measuring overall immunosuppressive load and association with PTLD risk and identifying new risk factors (HLA associated? Non-EBV viruses?) |
| Pathogenesis | Providing comprehensive overview of genomic landscape by using next-generation sequencing (both EBV+ and EBV− PTLD) |
| Diagnosis | Refining WHO 2008 classification, including impact of EBV (negative, positive, latency type, lytic activation), stromal microenvironment, and molecular genetic findings |
| Staging and response assessment | Determining role of hybrid PET/magnetic resonance imaging, other tracers (eg, 18F-fluoro-3′-deoxythymidine), and immuno-PET |
| Prevention | Improving preemptive strategies (eg, EBV PCR, cytokine gene polymorphisms, and chemokine (C-X-C motif) ligand 13) |
| Treatment | Aiming for international cooperation and inclusion of patients in prospective international trials |
| Prognosis | Identifying new (clinical and nonclinical) prognostic factors and factors predictive for response, aiming to identify patients with poor outcome on “standard” therapy and provide an opportunity for risk-adapted treatment strategies in the future |
| Aspect . | Research/clinical challenges/opportunities . |
|---|---|
| Incidence | Integrating large registries and complete multicenter/nationwide detailed information, preferentially prospective |
| Risk factors | Searching for tools measuring overall immunosuppressive load and association with PTLD risk and identifying new risk factors (HLA associated? Non-EBV viruses?) |
| Pathogenesis | Providing comprehensive overview of genomic landscape by using next-generation sequencing (both EBV+ and EBV− PTLD) |
| Diagnosis | Refining WHO 2008 classification, including impact of EBV (negative, positive, latency type, lytic activation), stromal microenvironment, and molecular genetic findings |
| Staging and response assessment | Determining role of hybrid PET/magnetic resonance imaging, other tracers (eg, 18F-fluoro-3′-deoxythymidine), and immuno-PET |
| Prevention | Improving preemptive strategies (eg, EBV PCR, cytokine gene polymorphisms, and chemokine (C-X-C motif) ligand 13) |
| Treatment | Aiming for international cooperation and inclusion of patients in prospective international trials |
| Prognosis | Identifying new (clinical and nonclinical) prognostic factors and factors predictive for response, aiming to identify patients with poor outcome on “standard” therapy and provide an opportunity for risk-adapted treatment strategies in the future |
Diagnosis of PTLD is not always straightforward, requiring refinement of the current WHO 2008 classification. This refinement will likely be possible only by incorporating findings derived from the microenvironment and by introducing molecular genetic characteristics. Although we and others have speculated, on the basis of transcriptomic and genomic studies, that EBV+ and EBV− PTLD seem to be different entities and, hence, should not be treated the same way, clinical data do not completely support this hypothesis, providing a strong rationale for well-designed clinical trials that avoid confounding factors.
RIS is considered the cornerstone of treatment in PTLD after SOT, although in most cases, additional therapy is warranted. Improved outcome observed with the use of rituximab in different B-cell NHLs has led to the introduction of this monoclonal antibody in several small (and 1 large multicenter) prospective phase 2 trials in PTLD. Results of these trials have established rituximab as the standard of care (either in monotherapy or followed by systemic chemotherapy) in most CD20+ PTLDs. Despite these improvements, survival of PTLD patients remains inferior, necessitating further international cooperation aimed at improving long-term outcome of PTLD patients. In addition, new and tolerable therapies are needed in the treatment of patients with PTLD. On the basis of in vitro observations, possible candidates include phosphatidylinositol 3-kinase inhibitors and mammalian target of rapamycin inhibitors.101,102 Whether checkpoint inhibitors can be used safely in PTLD has not yet been investigated, but a major obstacle will be the increased risk for graft rejection, which is exemplified by the fact that CTLA4-immunoglobulin (belatacept) is approved for prevention of graft rejection in kidney transplants at the expense of an increased risk for PTLD development.103 Inversely, it can be expected that CTLA4 inhibitors might be used in the treatment of PTLD, although with an increased risk for graft rejection.
Acknowledgments
The authors thank the other members of the Leuven PTLD Group (Prof Gregor Verhoef, Dr Julie Morscio, Prof Iwona Wlodarska, and Dr Marleen Renard) for their assistance in preparing the manuscript and for their critical review of the final version of the manuscript.
This work was supported by a research grant (G081411N) from Fonds Wetenschappelijk Onderzoek –Vlaanderen and by a concerted action grant (3M040406) from Catholic University Leuven (T.T.). T.T. holds a Mandate for Fundamental and Translational Research from the Stichting tegen Kanker (2014-083); D.D. holds a Mandate for Clinical Research from the University Hospitals Leuven and is a cofounder of Stefanie’s Rozen Fonds, Fonds Tom Debackere voor lymfoomonderzoek, and Fonds Jos en Mieke Vandevordt-Gaul voor de pathogenese van zeldzame lymfomen; and O.G. is a senior clinical investigator of Fonds Wetenschappelijk Onderzoek–Vlaanderen.
Authorship
Contribution: D.D., T.T., and O.G. wrote the manuscript; all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daan Dierickx, Department of Hematology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium; e-mail: daan.dierickx@uzleuven.be.
References
Author notes
D.D., T.T., and O.G. contributed equally to this study.