Key Points
This is the first clinical trial to investigate CPD in multiple myeloma.
Results suggest that the regimen is a well-tolerated and highly active combination for patients with relapsed/refractory multiple myeloma.
Abstract
Treatment options for patients with heavily pretreated relapsed and/or refractory multiple myeloma remain limited. We evaluated a novel therapeutic regimen consisting of carfilzomib, pomalidomide, and dexamethasone (CPD) in an open-label, multicenter, phase 1, dose-escalation study. Patients who relapsed after prior therapy or were refractory to the most recently received therapy were eligible. All patients were refractory to prior lenalidomide. Patients received carfilzomib IV on days 1, 2, 8, 9, 15, and 16 (starting dose of 20/27 mg/m2), pomalidomide once daily on days 1 to 21 (4 mg as the initial dose level), and dexamethasone (40 mg oral or IV) on days 1, 8, 15, and 22 of 28-day cycles. The primary objective was to evaluate the safety and determine the maximum tolerated dose (MTD) of the regimen. A total of 32 patients were enrolled. The MTD of the regimen was dose level 1 (carfilzomib 20/27 mg/m2, pomalidomide 4 mg, dexamethasone 40 mg). Hematologic adverse events (AEs) occurred in ≥60% of all patients, including 11 patients with grade ≥3 anemia. Dyspnea was limited to grade 1/2 in 10 patients. Peripheral neuropathy was uncommon and limited to grade 1/2. Eight patients had dose reductions during therapy, and 7 patients discontinued treatment due to AEs. Two deaths were noted on study due to pneumonia and pulmonary embolism (n = 1 each). The combination of CPD is well-tolerated and highly active in patients with relapsed/refractory multiple myeloma. This trial was registered at www.clinicaltrials.gov as #NCT01464034.
Introduction
The management of relapsed/refractory multiple myeloma (RRMM) has changed significantly in the last 10 years with the introduction of new agents and combinations with improved efficacy and more favorable adverse event (AE) profiles, including thalidomide, lenalidomide/dexamethasone, bortezomib/lenalidomide/dexamethasone, bortezomib/cyclophosphamide/dexamethasone, and bortezomib/pegylated liposomal doxorubicin.1-11 Results from clinical trials have also demonstrated the feasibility of the combination of a proteasome inhibitor, an immunomodulatory agent (IMiD), and dexamethasone with encouraging efficacy in both newly diagnosed myeloma and RRMM.12-15 Unfortunately, not all patients respond to bortezomib- or lenalidomide-based therapy in the relapsed setting, and continued use of these agents is universally limited by either treatment-emergent toxicity or refractory disease.
Two novel, second-in-class myeloma agents have recently been added to the myeloma armamentarium to address the need for further effective therapies. Carfilzomib, a selective and irreversible proteasome inhibitor, was approved in the United States for patients with disease refractory to the most recent therapy and after at least 2 prior therapies, including bortezomib and an IMiD, on the basis of a single-arm, phase 2 study of 257 response-evaluable patients that demonstrated an overall response rate (ORR) of 22.9%, a duration of response of 7.8 months, and a median overall survival (OS) of 15.6 months.16-19 Based on an ORR of 20% to 30% in the RRMM setting, the IMiD pomalidomide in combination with dexamethasone has been approved for patients with RRMM who have progressed after receiving at least 2 prior lines of therapy, including lenalidomide and bortezomib, and specifically in patients with lenalidomide-refractory disease.20 A recently described mechanism of action of lenalidomide includes increased protein ubiquitination and brings into question mechanism of synergy between IMiDs and proteasome inhibitors.21,22 Despite this paradoxical preclinical data, clinical experience supports their synergistic activity.6 Therefore, we hypothesized that the combination of carfilzomib and pomalidomide would yield a safe and active therapy for patients with RRMM. Herein, we report results of a multicenter, phase 1, dose-escalation trial of carfilzomib, pomalidomide, and dexamethasone (CPD) in patients with RRMM.
Methods
Patient selection
Patients were required to have had a confirmed diagnosis of multiple myeloma that was relapsed after prior therapy or that was refractory to the most recently received therapy. All patients must have received prior lenalidomide therapy and must have been refractory to a regimen containing a full (25 mg) or maximum tolerated dose (MTD) of lenalidomide. Patients progressing on low-dose lenalidomide maintenance were not eligible to enroll. Key inclusion criteria included: age ≥18 years; Eastern Cooperative Oncology Group performance status of 0 to 2; adequate hepatic function, with bilirubin <2 times the upper limit of normal, and aspartate aminotransferase and alanine aminotransferase <3 times the upper limit of normal; serum creatinine ≤2 mg/dL or creatinine clearance ≥50 mL per minute; and adequate bone marrow function as evidenced by an absolute neutrophil count ≥1.0 × 109/L, a hemoglobin level ≥8 g/dL, and a platelet count ≥50 × 109/L. Exclusion criteria included: congestive heart failure (CHF; New York Heart Association class III-IV), symptomatic ischemia, conduction system abnormalities uncontrolled by conventional intervention, myocardial infarction in the previous 6 months, or uncontrolled hypertension, and significant neuropathy (grade 3/4, or grade 2 with pain). Patients must also have provided written informed consent. Patients had to commit to standard contraceptive guidelines during pomalidomide therapy.
The study was conducted in accordance with the Declaration of Helsinki and the guidelines for good clinical practice.
Study design
This was a multicenter, phase 1, open-label, dose-finding study that was designed to evaluate the safety and to determine the MTD of CPD in patients with RRMM. Secondary objectives were to evaluate the preliminary efficacy of CPD measuring the ORR (ie, complete response [CR], very-good-partial response [VGPR], partial response [PR], or minimal response [MR]), time to progression, progression-free survival (PFS), and OS.
Patients were evaluated for dose-limiting toxicity (DLT) according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. A DLT was defined as any of the following treatment-emergent toxicities that were attributable to at least 1 of the study drugs that occurred during cycle 1. Nonhematologic DLTs included: (1) grade 2 treatment-emergent neuropathy with pain or grade ≥3 neuropathy; (2) any grade ≥3 toxicity (excluding nausea, vomiting, diarrhea); (3) grade ≥3 nausea, vomiting, or diarrhea despite maximal antiemetic/antidiarrheal therapy; (4) any nonhematologic toxicity requiring a dose reduction or dose hold within cycle 1; and (5) a delay in the ability to receive the day 1 dose of cycle 2 due to drug-related toxicity that persisted from cycle 1 >14 days. Hematologic DLTs included: (1) grade 4 neutropenia lasting for ≥7 days; (2) febrile neutropenia; (3) grade 4 thrombocytopenia lasting ≥7 days despite dose delay; (4) grade 3-4 thrombocytopenia associated with bleeding; (5) any hematologic toxicity requiring a dose reduction or dose hold within cycle 1; (6) an inability to receive the day 1 dose of cycle 2 owing to drug-related toxicity that persisted from cycle >14 days from the end of cycle 1.
There was a mandatory deep vein thrombosis (DVT) prophylaxis, which was either aspirin (81 mg) daily or, for those with prior history of DVT, full anticoagulation treatment.
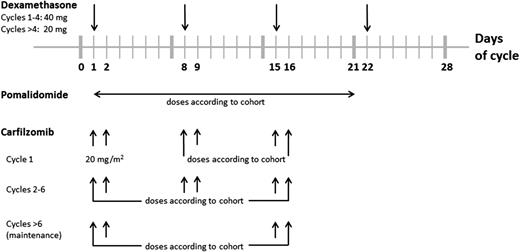
At least 48 hours before cycle 1 day 1, patients had to have oral hydration of 30 mL/kg per day (∼6-8 cups of liquid per day) continuing up to the time of treatment. Additional IV hydration (250-500 mL normal saline or other appropriate IV fluid) was given immediately before and after each carfilzomib dose during cycle 1. Carfilzomib was dosed on days 1 and 2 of cycle 1 for all cohorts at a starting dose of 20 mg/m2; all subsequent doses, starting with cycle 1 day 8, were administered at a higher dose that was assigned per cohort level (refer to Table 1). Carfilzomib was administered as an IV infusion over 30 minutes on days 1, 2, 8, 9, 15, and 16, every 28 days for the first 6 cycles. Pomalidomide was dosed orally once daily on days 1 to 21, every 28 days (refer to Table 1 and Figure 1). Dexamethasone (40 mg) was administered weekly (oral or IV) on days 1, 8, 15, and 22, every 28 days. After the first 4 cycles, it was recommended that the dose of dexamethasone be reduced to 20 mg. After completing 6 cycles of treatment, patients could proceed to the maintenance phase of the study, during which the carfilzomib dosing was to occur on days 1, 2, 15, and 16, every 28 days, and the pomalidomide dosing was to remain on days 1 to 21, every 28 days. If disease progression occurred on maintenance dosing, patients could resume full dosing and add back the day 8 and 9 doses of carfilzomib. Patients continued on treatment as long as they were receiving clinical benefit.
Planned dosing cohorts
| Cohort . | Carfilzomib,* mg/m2 . | Pomalidomide, mg . | Dexamethasone,† mg . |
|---|---|---|---|
| −3 | 20 | 2 | 40 |
| −2 | 27 | 2 | 40 |
| −1 | 27 | 3 | 40 |
| 1 (initial dose level) | 27 | 4 | 40 |
| 2 | 36 | 4 | 40 |
| 3 | 45 | 4 | 40 |
| 4 | 56 | 4 | 40 |
| Cohort . | Carfilzomib,* mg/m2 . | Pomalidomide, mg . | Dexamethasone,† mg . |
|---|---|---|---|
| −3 | 20 | 2 | 40 |
| −2 | 27 | 2 | 40 |
| −1 | 27 | 3 | 40 |
| 1 (initial dose level) | 27 | 4 | 40 |
| 2 | 36 | 4 | 40 |
| 3 | 45 | 4 | 40 |
| 4 | 56 | 4 | 40 |
Carfilzomib was administered IV over 30 minutes; pomalidomide was administered orally; dexamethasone was administered either IV or orally.
The first 2 doses of carfilzomib in cycle 1 were to be administered at 20 mg/m2.
Dosing of dexamethasone may be modified at the discretion of the treating investigator.
A standard 3 + 3 dose-escalation schedule was used in the study, starting at cohort 1 with up to 4 sequential dose-escalating cohorts and/or 3 dose-de-escalating cohorts with 3 to 6 patients in each cohort. If fewer than 24 patients were enrolled during the dose-escalation phase, additional patients were to be enrolled into the trial at the MTD, with up to a maximum of 30 patients evaluable for toxicity. All patients were considered evaluable for toxicity unless they could not complete the first cycle of therapy owing to withdrawal of consent or disease progression. Patients must have completed 2 cycles of therapy to be evaluable for efficacy unless a patient was removed from study prior to completing 2 cycles owing to disease progression, in which case they would still be considered evaluable for response.
Response and safety assessment/criteria
Responses were assessed on cycle 1 day 15 and on day 1 of all subsequent cycles. Responses and progression were assessed by the investigators and then confirmed by the principal investigator according to the uniform response criteria of the International Myeloma Working Group: stringent CR, CR, VGPR, PR, stable disease, and progressive disease, with minimal response as defined by the European Bone Marrow Transplant criteria.23,24 The ORR was defined as the percentage of patients who achieved at least a PR, and the clinical benefit rate (CBR) was defined as those patients who achieved a minimum of MR. Neurologic assessments were performed at the beginning of each cycle using the questionnaire from the neurotoxicity subscale of the Gynecologic Oncology Group’s Functional Assessment of Cancer Therapy.
Statistical measures
Safety analyses were conducted in all patients who received at least 1 dose of study treatment. Patient demographic and clinical characteristics at baseline were summarized using descriptive statistics. The Student t test/Wilcoxon test and the analysis of variance/Kruskal-Wallis test were used to compare continuous variables between different patient groups. The χ2 test or the Fisher exact test was applied to assess the association between 2 categorical variables. Time-to-event outcomes, including PFS and OS, were estimated using the Kaplan-Meier method. Toxicities were summarized by tabulating the maximum grade for each unique AE per patient.
Results
Patients
A total of 32 patients with RRMM were enrolled at 8 centers in the United States. One patient withdrew consent during cycle 1 after only 2 doses of carfilzomib and subsequently developed rapidly progressive disease and hypercalcemia. The second patient was removed from the study for hypercalcemia and renal dysfunction unrelated to study drug after receiving day 1 and 2 carfilzomib and 4 doses of pomalidomide. Although these 2 patients did not receive adequate therapy, they were included in the intent-to-treat response evaluation and in the DLT evaluation.
All remaining patients received 2 or more full cycles of study therapy. All patients were refractory to full-dose lenalidomide, 31 of 32 (97%) had received prior bortezomib, and all but 2 (91%) were refractory to prior bortezomib (see Table 2). Twenty-one patients (67%) received prior autologous stem cell transplant. The median number of lines of prior therapy received by patients was 6 (range, 2-12).
Baseline patient demographics, disease, and treatment characteristics
| . | N = 32 . |
|---|---|
| Mean age, median (range), y | 64 (44-78) |
| Sex, no. (%) | |
| Male | 20 (62.5) |
| Female | 12 (37.55) |
| ECOG performance status, no. (%) | |
| 0 | 16 (53) |
| 1 | 12 (40) |
| 2 | 2 (7) |
| Time since initial diagnosis, y (range) | 5.9 (1.2-13) |
| Prior regimens, median (range) | 6 (2-12) |
| Prior therapies, no. (%) | |
| Transplant | 21 (67) |
| Bortezomib | 31 (97; all but 2 bortezomib refractory) |
| Lenalidomide | 32 (100; all refractory) |
| Cytogenetics | |
| Hypodiploid | 3 |
| Hyperdiploid | 10 |
| Del(1) | 3 |
| Del(13) | 9 |
| Del(17p) | 5 |
| t(4;14) | 3 |
| t(11;14) | 5 |
| t(14;16) | 2 |
| . | N = 32 . |
|---|---|
| Mean age, median (range), y | 64 (44-78) |
| Sex, no. (%) | |
| Male | 20 (62.5) |
| Female | 12 (37.55) |
| ECOG performance status, no. (%) | |
| 0 | 16 (53) |
| 1 | 12 (40) |
| 2 | 2 (7) |
| Time since initial diagnosis, y (range) | 5.9 (1.2-13) |
| Prior regimens, median (range) | 6 (2-12) |
| Prior therapies, no. (%) | |
| Transplant | 21 (67) |
| Bortezomib | 31 (97; all but 2 bortezomib refractory) |
| Lenalidomide | 32 (100; all refractory) |
| Cytogenetics | |
| Hypodiploid | 3 |
| Hyperdiploid | 10 |
| Del(1) | 3 |
| Del(13) | 9 |
| Del(17p) | 5 |
| t(4;14) | 3 |
| t(11;14) | 5 |
| t(14;16) | 2 |
ECOG, Eastern Cooperative Oncology Group.
Dose escalation
In the first dosing cohort (carfilzomib 20/27 mg/m2, pomalidomide 4 mg, dexamethasone 40 mg), 1 of 6 patients encountered a protocol-defined DLT of febrile neutropenia. The patient presented with a fever and a nadir absolute neutrophil count of 980 cells per μL, was diagnosed with community-acquired pneumonia, defervesced in 24 hours, and completed an uncomplicated course of outpatient antibiotics. At the second dose level (carfilzomib 20/36 mg/m2, pomalidomide 4 mg, and dexamethasone 40 mg), 2 of 6 patients experienced a DLT. The first patient had grade 4 thrombocytopenia, and the second patient experienced a grade 3 rash during cycle 1 owing to pomalidomide; this patient had a minor rash with prior lenalidomide. Because of these 2 DLTs, the MTD was determined to be dose level 1 (ie, carfilzomib 20/27 mg/m2, pomalidomide 4 mg, and dexamethasone 40 mg). Twelve patients were treated in the dose-escalation phase, and 20 additional patients were enrolled at the MTD to further establish the safety profile and to assess the preliminary activity of CPD.
Toxicity
Of the 32 enrolled patients, 28 had either an AE related to the study treatment or an infection (Table 3). A total of 20 of 32 patients (63%) had any grade 3 event; 10 of 32 patients (31%) had grade 4 events, and 2 (6%) had a fatal event (pneumonia and pulmonary embolism [n = 1 each]).
Adverse events, by maximum grade reported
| . | Grade . | Total (N = 32) . | ||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ||
| Hematologic AEs, n | ||||||
| Anemia | 0 | 4 | 5 | 1 | 0 | 10 |
| Thrombocytopenia | 0 | 3 | 4 | 3 | 0 | 10 |
| Neutropenia | 0 | 4 | 10 | 4 | 0 | 18 |
| Febrile neutropenia | 0 | 0 | 2 | 0 | 0 | 2 |
| Nonhematologic AEs, n | ||||||
| Diarrhea | 4 | 1 | 0 | 0 | 0 | 5 |
| Constipation | 6 | 0 | 0 | 0 | 0 | 6 |
| Nausea | 1 | 1 | 1 | 0 | 0 | 3 |
| Emesis | 0 | 1 | 1 | 0 | 0 | 2 |
| Fatigue | 5 | 9 | 1 | 0 | 0 | 15 |
| Dyspnea | 8 | 2 | 1 | 0 | 0 | 8 |
| Rash | 4 | 0 | 1 | 0 | 0 | 5 |
| Neuropathy/paresthesia | 6 | 1 | 0 | 0 | 0 | 6 |
| CHF | 0 | 0 | 1 | 0 | 0 | 1 |
| Muscle spasms | 4 | 2 | 0 | 0 | 0 | 6 |
| Transient ischemic attack | 0 | 0 | 1 | 0 | 0 | 1 |
| PE | 0 | 0 | 1 | 0 | 1 | 2 |
| DVT | 0 | 1 | 0 | 0 | 0 | 1 |
| Creatinine elevation | 5 | 4 | 1 | 2 | 0 | 12 |
| Skin infection | 2 | 0 | 1 | 0 | 0 | 3 |
| Pneumonia | 0 | 0 | 3 | 0 | 1 | 4 |
| Pneumonia parainfluenza viral | 2 | 0 | 0 | 0 | 0 | 1 |
| Upper respiratory tract infection | 1 | 0 | 0 | 0 | 0 | 1 |
| Alanine aminotransferase increased | 3 | 0 | 0 | 0 | 0 | 3 |
| Aspartate aminotransferase increased | 2 | 1 | 0 | 0 | 0 | 3 |
| Hyperbilirubinemia | 2 | 0 | 0 | 0 | 0 | 2 |
| Hypocalcemia | 2 | 3 | 0 | 0 | 0 | 5 |
| . | Grade . | Total (N = 32) . | ||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ||
| Hematologic AEs, n | ||||||
| Anemia | 0 | 4 | 5 | 1 | 0 | 10 |
| Thrombocytopenia | 0 | 3 | 4 | 3 | 0 | 10 |
| Neutropenia | 0 | 4 | 10 | 4 | 0 | 18 |
| Febrile neutropenia | 0 | 0 | 2 | 0 | 0 | 2 |
| Nonhematologic AEs, n | ||||||
| Diarrhea | 4 | 1 | 0 | 0 | 0 | 5 |
| Constipation | 6 | 0 | 0 | 0 | 0 | 6 |
| Nausea | 1 | 1 | 1 | 0 | 0 | 3 |
| Emesis | 0 | 1 | 1 | 0 | 0 | 2 |
| Fatigue | 5 | 9 | 1 | 0 | 0 | 15 |
| Dyspnea | 8 | 2 | 1 | 0 | 0 | 8 |
| Rash | 4 | 0 | 1 | 0 | 0 | 5 |
| Neuropathy/paresthesia | 6 | 1 | 0 | 0 | 0 | 6 |
| CHF | 0 | 0 | 1 | 0 | 0 | 1 |
| Muscle spasms | 4 | 2 | 0 | 0 | 0 | 6 |
| Transient ischemic attack | 0 | 0 | 1 | 0 | 0 | 1 |
| PE | 0 | 0 | 1 | 0 | 1 | 2 |
| DVT | 0 | 1 | 0 | 0 | 0 | 1 |
| Creatinine elevation | 5 | 4 | 1 | 2 | 0 | 12 |
| Skin infection | 2 | 0 | 1 | 0 | 0 | 3 |
| Pneumonia | 0 | 0 | 3 | 0 | 1 | 4 |
| Pneumonia parainfluenza viral | 2 | 0 | 0 | 0 | 0 | 1 |
| Upper respiratory tract infection | 1 | 0 | 0 | 0 | 0 | 1 |
| Alanine aminotransferase increased | 3 | 0 | 0 | 0 | 0 | 3 |
| Aspartate aminotransferase increased | 2 | 1 | 0 | 0 | 0 | 3 |
| Hyperbilirubinemia | 2 | 0 | 0 | 0 | 0 | 2 |
| Hypocalcemia | 2 | 3 | 0 | 0 | 0 | 5 |
Hematologic AEs are listed in Table 3. Five patients (16%) received platelets transfusion (n = 1 each) and 19 patients (59%) required at least 1 transfusion of red blood cells (median, 1; range, 0-13). Grade ≥3 nonhematologic AEs of interest included 1 patient each with grade 3 CHF, fluid overload, muscle weakness, maculopapular rash, and dyspnea, 1 patient with grade 3 acute renal failure, and 2 patients with grade 4 acute renal failure. Two patients had a pulmonary embolism (with 1 grade 5 event), both were on aspirin prophylaxis prior to the event. Infections included 3 patients with grade 3 pneumonia and 1 patient with grade 5 pneumonia. Treatment-emergent peripheral neuropathy was uncommon, with 6 patients experiencing grade 1 peripheral neuropathy and 1 patient experiencing grade 2 peripheral neuropathy. Two deaths were noted on study due to pneumonia and pulmonary embolism (PE).
Twelve patients had dose reductions of carfilzomib or pomalidomide during therapy (Table 4). Five patients had a dose reduction of carfilzomib in cycles 3, 5 (n = 2), 6, 10, and 13 owing to pneumonia, weight loss, shortness of breath, CHF, and neutropenia, respectively. One of those patients had carfilzomib dose reduced in cycles 3 and 13 due to pneumonia and neutropenia, respectively. Seven patients had pomalidomide dose reductions during cycles 1, 2 (n = 4), 3, 4, 5, and 6 owing to febrile neutropenia/neutropenia (n = 4), thrombocytopenia (n = 2), anemia, rash, and a car accident-related hospitalization. One of those patients had pomalidomide dose reduced in cycles 1 and 2 due to febrile neutropenia and in cycle 6 due to a car accident. No patients had dose reductions during the maintenance phase.
Dose reductions and discontinuations
| Cycle . | No. of patients (%) . | Dose reductions of carfilzomib . | Dose reductions of pomalidomide . | Dose discontinuations of pomalidomide or carfilzomib . | ||
|---|---|---|---|---|---|---|
| n . | Reason . | n . | Reason . | |||
| 1 | 32 (100) | — | — | 1 | Febrile neutropenia | 4, includes 1 discontinuation due to an AE or SAE, 1 discontinuation due to patient’s death, and 1 withdrawal of consent |
| 2 | 28 (89) | — | — | 4 | Febrile neutropenia, rash, neutropenia, thrombocytopenia | 1 |
| 3 | 27 (84) | 1 | Pneumonia | 1 | Thrombocytopenia | 2, includes 1 discontinuation due to an AE or SAE |
| 4 | 25 (78) | — | — | 1 | Anemia | 4 |
| 5 | 21 (66) | 2 | Weight loss, shortness of breath | 1 | Neutropenia | 2 |
| 6 | 19 (59) | 1 | CHF | 1 | Car accident | 2, includes 1 discontinuation due to patient’s death |
| 7 | 17 (53) | — | — | — | — | 3, includes 1 withdrawal of consent |
| 8 | 15 (47) | — | — | — | — | 3, includes 1 discontinuation due to an AE or SAE and 1 withdrawal of consent |
| 9 | 12 (38) | — | — | — | — | 2 |
| 10 | 9 (28) | 1 | Neutropenia | — | — | 2 |
| 11 | 7 (22) | — | — | — | — | 2, includes 1 discontinuation due to patient’s death |
| 12 | 5 (16) | — | — | — | — | — |
| 13 | 5 (16) | 1 | Neutropenia | — | — | — |
| 14 | 5 (16) | — | — | — | — | 2 |
| 15 | 3 (9) | — | — | — | — | — |
| 16 | 3 (9) | — | — | — | — | — |
| 17 | 3 (9) | — | — | — | — | — |
| 18+ | 3 (9) | — | — | — | — | 3, includes 1 discontinuation due to an AE or SAE |
| Cycle . | No. of patients (%) . | Dose reductions of carfilzomib . | Dose reductions of pomalidomide . | Dose discontinuations of pomalidomide or carfilzomib . | ||
|---|---|---|---|---|---|---|
| n . | Reason . | n . | Reason . | |||
| 1 | 32 (100) | — | — | 1 | Febrile neutropenia | 4, includes 1 discontinuation due to an AE or SAE, 1 discontinuation due to patient’s death, and 1 withdrawal of consent |
| 2 | 28 (89) | — | — | 4 | Febrile neutropenia, rash, neutropenia, thrombocytopenia | 1 |
| 3 | 27 (84) | 1 | Pneumonia | 1 | Thrombocytopenia | 2, includes 1 discontinuation due to an AE or SAE |
| 4 | 25 (78) | — | — | 1 | Anemia | 4 |
| 5 | 21 (66) | 2 | Weight loss, shortness of breath | 1 | Neutropenia | 2 |
| 6 | 19 (59) | 1 | CHF | 1 | Car accident | 2, includes 1 discontinuation due to patient’s death |
| 7 | 17 (53) | — | — | — | — | 3, includes 1 withdrawal of consent |
| 8 | 15 (47) | — | — | — | — | 3, includes 1 discontinuation due to an AE or SAE and 1 withdrawal of consent |
| 9 | 12 (38) | — | — | — | — | 2 |
| 10 | 9 (28) | 1 | Neutropenia | — | — | 2 |
| 11 | 7 (22) | — | — | — | — | 2, includes 1 discontinuation due to patient’s death |
| 12 | 5 (16) | — | — | — | — | — |
| 13 | 5 (16) | 1 | Neutropenia | — | — | — |
| 14 | 5 (16) | — | — | — | — | 2 |
| 15 | 3 (9) | — | — | — | — | — |
| 16 | 3 (9) | — | — | — | — | — |
| 17 | 3 (9) | — | — | — | — | — |
| 18+ | 3 (9) | — | — | — | — | 3, includes 1 discontinuation due to an AE or SAE |
SAE, severe AE.
— indicates no reduction or discontinuation.
All patients are off study, and the majority of patients (22) discontinued therapy for disease progression; 6 patients discontinued owing to AEs; and 3 patients withdrew consent owing to plans to proceed to autologous stem cell transplant, an inability to travel for therapy, and patient preference, respectively. The AEs leading to discontinuation were renal failure (n = 3), pulmonary emboli with death (n = 1), and pneumonia (n = 2 with 1 death).
Efficacy
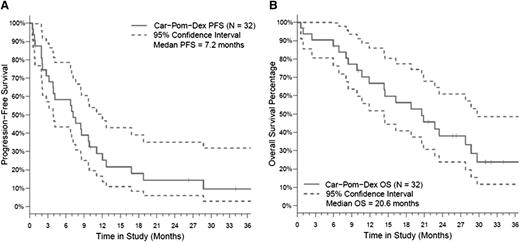
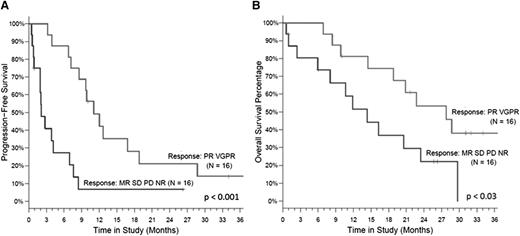
All 32 patients were included in the intent-to-treat response assessment. Patients received a median of 7 cycles (range, 1-24) of CPD. The ORR was 50%, and the CBR (≥MR) was 66% with 5 of 32 patients (16%) achieving a VGPR, 11 of 32 patients (34%) achieving PR, 5 of 32 patients (16%) with MR, 8 of 32 patients (25%) with stable disease (Table 5). Seventeen patients started the maintenance phase in cycle 7 (16 with dose-reduced maintenance dosing and 1 continuing at full dose with the inclusion of day 8 and 9 carfilzomib). After a median follow-up of 26.3 months (range, 1-37), the median PFS was 7.2 months (95% confidence interval, 3-9 months). The median OS was 20.6 months (95% confidence interval, 11.9-28.7 months), with a 12-month OS rate of 67% (Figure 2). Patients who reached PR or VGPR had a significantly better PFS and OS than those with responses less than PR, P < .001 and .03, respectively (Figure 3).
Response rate in all intent-to-treat patients and by cytogenetic abnormalities
| Response category, n (%) . | All evaluable patients, N = 32 . | Hyperdiploid, n = 10 . | Del(13), n = 9 . | Del(17p), n = 5 . |
|---|---|---|---|---|
| ORR | 16 (50) | 5 (50) | 1(11) | 4 (80) |
| VGPR | 5 (16) | 1 (10) | 1(11) | 1 (20) |
| PR | 11 (34) | 4 (40) | 0 | 3 (60) |
| MR | 5 (16) | 3 (30) | 3 (33) | 0 |
| SD | 8 (25) | 2 (20) | 5 (56) | 1 (20) |
| PD | 3 (9) | 0 | 0 | 0 |
| Response category, n (%) . | All evaluable patients, N = 32 . | Hyperdiploid, n = 10 . | Del(13), n = 9 . | Del(17p), n = 5 . |
|---|---|---|---|---|
| ORR | 16 (50) | 5 (50) | 1(11) | 4 (80) |
| VGPR | 5 (16) | 1 (10) | 1(11) | 1 (20) |
| PR | 11 (34) | 4 (40) | 0 | 3 (60) |
| MR | 5 (16) | 3 (30) | 3 (33) | 0 |
| SD | 8 (25) | 2 (20) | 5 (56) | 1 (20) |
| PD | 3 (9) | 0 | 0 | 0 |
PD, progressive disease; SD, stable disease.
Survival in the overall study population. (A) PFS and (B) OS in the overall study population (n = 32).
Survival in the overall study population. (A) PFS and (B) OS in the overall study population (n = 32).
The efficacy was observed in all cytological abnormalities (Table 5). Interestingly, among the 5 patients with deletion of the short arm of chromosome 17, the 12-month PFS rate was 60% and the 12-month OS rate was 80%, which were better than the rates observed for the entire cohort (29% and 67%, respectively; Figure 2). However, numbers are low for a definite conclusion and, thus, the comparative efficacy is being assessed in an expanded cohort.
Discussion
Based on strong preclinical and clinical evidence supporting combinations of proteasome inhibitors with immunomodulatory agents for myeloma therapy, we conducted a phase 1 trial combining 2 novel agents. We were able to demonstrate that CPD is a well-tolerated and highly active regimen. The MTD of CPD in this heavily pretreated patient population (median of 6 lines of prior therapy) was determined to be carfilzomib 20/27 mg/m2, pomalidomide 4 mg, and dexamethasone 40 mg.
Importantly, grade ≥3 nonhematologic toxicities were uncommon; notable toxicities were 1 CHF, 2 PEs with 1 death, 3 renal failures, and 4 cases of pneumonia with 1 death. Although dyspnea occurred frequently, it was limited to grade 1 and 2 events. The incidence rates of these AEs were similar to those reported by phase 1 trials of carfilzomib/lenalidomide/dexamethasone in RRMM.25 Patients were able to receive a median of 7.7 cycles of therapy, and the regimen was well tolerated, with only 7 patients discontinuing therapy owing to toxicity.
Other carfilzomib-based regimens, including those in combination with lenalidomide, thalidomide, panobinostat, and cyclophosphamide, have demonstrated the ability to administer higher doses of carfilzomib.26-30 Although such studies often enrolled slightly different populations of patients with less heavily pretreated refractory MM.
During the dose-escalation phase of the present study, we chose a conservative approach regarding DLTs, with 1 of the DLT events observed as a grade 3 rash in a patient who also had a rash following prior lenalidomide. The grade 3 rash limited the ability to dose escalate in our study, and as a result, the MTD may have been underestimated. In the future, a subsequent dose escalation is planned in less heavily pretreated patients (ie, 1-3 lines of therapy with prior lenalidomide exposure) to reevaluate the MTD of carfilzomib in this combination.
We observed a high response rate (ORR 50% and CBR 66%) despite enrolling patients who were uniformly refractory to full-dose lenalidomide and all but 2 were refractory to bortezomib as well. Notably, the median PFS was 7.2 months, and the median OS was 20.6 months. This compares favorably to historical controls of patients who were refractory to both IMiDs and bortezomib who had a median event-free survival of 5 months and a median OS of 9 months.31 The ORR and PFS also compare favorably with the activity of single-agent carfilzomib as well as pomalidomide ± dexamethasone in the MM003 trial, with ORRs ranging between 20% and 33% and a median PFS of 4 months.20,32-34
We also observed quality responses in patients with high-risk myeloma. This is consistent with a phase 2 trial presented by Leleu et al with pomalidomide/dexamethasone in patients with high-risk myeloma, specifically, del 17p and/or translocation t(4;14). In the phase 2 trial, the ORR was 27% in patients with del 17p and 16% with t(4;14), and it had greater benefit in patients with del 17 in regard to event-free survival and OS.35
In conclusion, at the MTD of carfilzomib 20/27 mg/m2, pomalidomide 4 mg, and dexamethasone 40 mg, the CPD regimen is a well-tolerated and highly active combination for patients with RRMM. The promising ORR and durable responses seen in patients, regardless of risk stratification, support further clinical trials of the combination in earlier lines of therapy as well as in high-risk myeloma. The combination may also provide a backbone for the incorporation of additional antimyeloma therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Medical writing and editorial assistance were provided by Christopher M. Brown, of BlueMomentum, an Ashfield Business, part of UDG Healthcare PLC, and funded by Onyx Pharmaceuticals, Inc, an Amgen subsidiary. Blue Momentum, funded by Onyx Pharmaceuticals, Inc, an Amgen subsidiary, assisted in final manuscript preparation for submission.
This work was supported by Onyx Pharmaceuticals, Inc, an Amgen subsidiary, and Celgene Corporation.
Authorship
Contribution: J.J.S. designed the trial, treated patients, analyzed the data, and wrote the manuscript; E.A.S., R.A., A.D.C., W.I.B., C.G., J.L.K., S.L., D.T.V., R.Z.O., and B.G.M.D. treated patients and reviewed/edited the manuscript; B.G.M.D., C.L.G., and N.P. designed the trial and edited the manuscript; and D.D.S. designed the trial, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: J.J.S. has received research funding from Array, Celgene, Millennium/Takeda, Novartis, and Onyx/Amgen. E.A.S. has consulted for Amgen, Celgene, Janssen, Novartis, and Takeda. R.A. has consulted for, served on speaker's bureau of, and received research funding from, Celgene, Takeda, and Onyx/Amgen. A.D.C. has served on advisory boards for Bristol-Myers Squibb, Janssen, Onyx, and Independent Response Adjudication Committee for Celgene, and has received research funding from Bristol-Myers Squibb. W.I.B. has served on advisory boards for Bristol-Myers Squibb, Celgene, Novartis, Sanofi, speaker's bureaus of Amgen and Celgene, and has received research funding from Acetylon, Amgen, Bristol-Myers Squibb, Celgene, Millenium/Takeda, Novartis, and Sanofi. C.G. has served on advisory boards for Celgene and Millennium/Takeda, has consulted for Celgene, Millennium/Takeda, and Onyx/Amgen, and has received honoraria from Celgene and Millennium/Takeda, and research funding from Celgene. J.L.K. has consulted for Celgene, Millennium/Takeda, Novartis, Onyx/Amgen, and Spectrum. S.L. has served on advisory boards or consulted for Bristol-Myers Squibb, Celgene, Janssen, and Novartis, and has served on speaker's bureau of Axiom. D.V.T. has consulted for Celgene and Onyx/Amgen, and has received research funding from Acetylon, Calithera, Constellation, GlaxoSmithKline, and Millennium/Takeda. R.Z.O. has served on advisory boards for, and has research funding support from, Celgene and Onyx/Amgen. B.G.M.D. has consulted for Bristol-Myers Squibb, Celgene, J&J, Millennium/Takeda, and Onyx/Amgen. The remaining authors declare no competing financial interests.
The current affiliation for A.D.C. is University of Pennsylvania Abramson Cancer Center, Philadelphia, PA.
Correspondence: Jatin J. Shah, Department of Lymphoma/Myeloma, MD Anderson Cancer Center, Unit 429, Houston, TX 77030; e-mail: jjshah@mdanderson.org.
Appendix: research in context
Evidence before this study
We searched the PubMed database for published articles to identify previous clinical trials in multiple myeloma that investigated the combination of CPD in the relapsed and/or refractory setting; no such studies were identified. However, given the known toxicity profiles of carfilzomib and pomalidomide, and the evidence that either agent could be safely combined with dexamethasone, we initiated this phase 1/2 study of CPD in patients with relapsed and/or refractory multiple myeloma.
Added value of this study
This is the first clinical trial to investigate CPD in multiple myeloma. Results suggest that the regimen is a well-tolerated and highly-active combination for patients.
Implications of all of the available evidence
The promising efficacy results observed in this study, regardless of patients’ risk stratification, support further clinical trials of CPD, both in advanced myeloma as well as in earlier lines of therapy.