Key Points
CD8+ T cells play a predominantly protective role in both passive and active murine models of ITP.
CD8+ T-regulatory cells contribute to efficacious response to steroid therapy and may be important diagnostic/prognostic biomarkers for ITP.
Abstract
Immune thrombocytopenia (ITP) is a common autoimmune bleeding disorder characterized by autoantibodies targeting platelet surface proteins, most commonly GPIIbIIIa (αIIbβ3 integrin), leading to platelet destruction. Recently, CD8+ cytotoxic T-lymphocytes (CTLs) targeting platelets and megakaryocytes have also been implicated in thrombocytopenia. Because steroids are the most commonly administered therapy for ITP worldwide, we established both active (immunized splenocyte engraftment) and passive (antibody injection) murine models of steroid treatment. Surprisingly, we found that, in both models, CD8+ T cells limited the severity of the thrombocytopenia and were required for an efficacious response to steroid therapy. Conversely, CD8+ T-cell depletion led to more severe thrombocytopenia, whereas CD8+ T-cell transfusion ameliorated thrombocytopenia. CD8+ T-regulatory cell (Treg) subsets were detected, and interestingly, dexamethasone (DEX) treatment selectively expanded CD8+ Tregs while decreasing CTLs. In vitro coculture studies revealed CD8+ Tregs suppressed CD4+ and CD19+ proliferation, platelet-associated immunoglobulin G generation, CTL cytotoxicity, platelet apoptosis, and clearance. Furthermore, we found increased production of anti-inflammatory interleukin-10 in coculture studies and in vivo after steroid treatment. Thus, we uncovered subsets of CD8+ Tregs and demonstrated their potent immunosuppressive and protective roles in experimentally induced thrombocytopenia. The data further elucidate mechanisms of steroid treatment and suggest therapeutic potential for CD8+ Tregs in immune thrombocytopenia.
Introduction
Immune thrombocytopenia or autoimmune thrombocytopenia (ITP) is a common bleeding disorder characterized by autoantibody-mediated destruction of platelets.1,2 Fatal intracranial hemorrhage can occur in severe cases (2% of patients).3,4 Autoantibodies targeting platelet glycoproteins (GPs) are considered to be the major mechanism behind platelet destruction by the reticuloendothelial system.5 The primary platelet surface antigen targets are GPIIbIIIa (αIIbβ3 integrin, 70-80% of patients).2,6,7 Approximately 10% to 20% of patients do not respond to first-line therapies including steroids,8,9 intravenous immunoglobulin G,10,11 and anti-Rh(D).4,10 Approximately 14% do not respond to splenectomy, and 20% of responders relapse within weeks to years.10,12,13 Reasons for refractoriness to therapy are unknown, and unfortunately, there are currently no reliable predictive factors to anticipate the success of any therapeutic regimen.
Development of more effective treatments is restricted by the current limited understanding of the immunopathogenesis of ITP.12,14-16 In addition to autoantibodies, other mechanisms of platelet destruction have been studied, the most significant of which is CD8+ cytotoxic T-lymphocyte (CTL)-mediated platelet destruction.17,18 Platelet-specific CTLs have been found to be elevated in patients with active ITP.19 In addition, CTL-mediated cytotoxicity of autologous platelets has been shown in vitro, whereby CTLs from ITP patients can cause direct platelet apoptosis or lysis.17,18 Further, in a murine model of ITP, transfusion of CD19+ B cell-depleted splenocytes, which contain CD8+ T cells, into SCID mice led to development of thrombocytopenia.20 CD8+ CTLs may also attack megakaryocytes in the bone marrow20 and affect platelet production. Thus, CD8+ T cells may play a significant pathogenic role in ITP.17,21,22
Immune dysregulation is central to the antiplatelet response.23 T-regulatory cells (Tregs), particularly CD4+CD25+FOXP3+ Tregs, play important roles in maintaining peripheral tolerance and have been shown to be dysfunctional or decreased in ITP patients.24-29 Recent studies suggest that successful therapies that raise platelet counts do so through normalization of CD4+ Tregs.30-32 In contrast to CD4+ Tregs, the study of CD8+ Tregs or T-suppressive cells, following their discovery in the 1970s,33-35 has fallen to the wayside. Technical limitations and conflicting evidence have led to difficulties with investigations into the functional properties of CD8+ Tregs. Recently, there has been a reemergence of the study of CD8+ Tregs and their roles in autoimmune diseases36-39 ; however, the role of CD8+ Tregs in ITP has not been investigated.
In this study, we developed 2 murine models of steroid treatment of ITP, which we termed “experimentally induced thrombocytopenia” (EIT). We found that, contrary to the current prevailing views of their pathogenic role (ie, CTL activity) in ITP, CD8+ T cells predominantly suppressed the pathogenesis and were required for effective steroid therapy in EIT. These results could potentially indicate that refractory patients may have a quantitative or functional deficit of CD8+ Tregs, which may serve as an indicative biomarker for response. Importantly, our study suggests that transfusion of CD8+ Treg cells may have therapeutic potential in ITP and deserves further investigation in human patients.
Methods
Animals
β3 integrin knockout (β3−/−) mice were kindly provided by Dr Richard O. Hynes (Massachusetts Institute of Technology, Boston. MA)40 and backcrossed 10 times to the BALB/c background as previously described.20,41-45 Syngeneic wild-type (WT) BALB/c mice (6-8 weeks) were purchased from Charles River Laboratories (Montreal, QC, Canada). All procedures were approved by the Animal Care Committee at St. Michael’s Hospital, Toronto, ON, Canada.
Passive ITP model of steroid therapy
As was previously described,46,47 WT BALB/c mice were injected intravenously via the tail vein on day 0 with 20 µL sera/mouse from immunized β3−/− mice or with anti-β3 monoclonal antibodies (mAbs) developed in our laboratory48,49 (Table 1) at a dose of 1 µg/mouse. Each mouse received DEX (D2915, 10 mg/kg intraperitoneally; Sigma-Aldrich) or phosphate-buffered saline (PBS) vehicle control beginning 4 hours after antibody injection and continuing daily thereafter. Platelets were enumerated daily for 4 days as previously described.41,44,50
Characterization of mouse anti-mouse mAb against β3 integrin
| Clone name . | IgG subtype . | Antigen . | Species cross-reactivity (tested) . |
|---|---|---|---|
| 9D2 | IgG 1 | β3 integrin | h, m, p, r |
| PSI C1 | IgG 1 | β3 integrin (PSI domain) | h, m, p, rb |
| JAN C1 | IgG 1 | β3 integrin | h, m, rb |
| Clone name . | IgG subtype . | Antigen . | Species cross-reactivity (tested) . |
|---|---|---|---|
| 9D2 | IgG 1 | β3 integrin | h, m, p, r |
| PSI C1 | IgG 1 | β3 integrin (PSI domain) | h, m, p, rb |
| JAN C1 | IgG 1 | β3 integrin | h, m, rb |
mAbs were generated in β3−/− mice. In addition to mouse (m), antibodies are also cross-reactive to other species, including human (h), pig (p), rat (r), and rabbit (rb).
Active ITP model of steroid therapy
Washed WT platelets were prepared as previously described.41,44 WT platelet immunization of β3−/− mice and the active murine model of ITP were established as previously described with minor modifications.20,41,42,44 Briefly, 5 × 106 splenocytes from immunized β3−/− mice were transfused into WT mice. For some groups, BALB/c recipient mice received 10 mg/kg per day of DEX orally through their drinking water beginning on day 6 and continuing for 1 to 2 weeks. Mouse water intake per day was calculated as previously described.51
For the indicated cell depletion studies, splenocytes were depleted of CD8+ T cells with the EasySep Magnetic cell sorting kit (StemCell Technologies) before engraftment. Depletion efficiencies were confirmed to be >90% by flow cytometry. Splenocyte-depleted CD8+ T cells (5 × 106) or splenocyte-depleted CD8+ T cells plus the enriched CD8+ T cells in the original ratio (8-10%) were transfused to WT recipients.
In vivo CD8+ T-cell depletion
Anti-CD8 mAb (400 μg; clone 2.43; ATCC) was injected intravenously to deplete CD8+ T cells from recipient mice before induction of thrombocytopenia. CD8+ T-cell count in the blood was quantified by flow cytometry to assess the extent of depletion.
CD8+ T-cell purification and transfusion
For in vitro suppression assays and adoptive transfer experiments, CD8+ T cells were enriched (>90%) using a CD8+ T-cell enrichment column (StemCell Technologies). CD8+ T cells (106) from WT, naïve β3−/−, or immunized β3−/− mice were transfused into thrombocytopenic mice.
Detection of CD8+ T cells by flow cytometry
Peripheral blood mononuclear cells and splenocytes from mice were incubated for 30 minutes at 22°C with the following antibodies: Peridinin chlorophyll (PerCP)-conjugated anti-mouse CD8, Sigma-Aldrich, PE-conjugated anti-mouse CD25, anti-mouse CD122, anti-mouse CD28, and anti-mouse CD103. Alternatively, the cells were incubated with PerCP-conjugated anti-mouse CD8, followed by Alex647-conjugated anti-mouse Foxp3 antibodies or PE-conjugated anti-mouse Perforin or PE-conjugated anti-mouse GranzymeB (Biolegend; 0.5 μg/mL). Cells were analyzed by flow cytometry.
Platelet apoptosis and clearance
After splenocytes were cocultured with autologous platelets and CD8+ T cells for 72 hours, platelet-rich supernatants were collected by centrifugation (300g, 5 minutes, 22°C), and platelets were collected by a second centrifugation (1250g, 15 minutes, 22°C) and then resuspended in 400 μL PBS. Platelets were incubated with PE-conjugated anti-CD41 mAb (Biolegend; 0.5 μg/mL). CD41+ platelets with identified and gated based on side and forward scatter characteristics using a log scale and further confirmed using a Beckman Z2 Coulter Counter.
To measure apoptosis, platelets were labeled with PE-conjugated anti-CD41 mAb, incubated with Annexin V-fluorescein isothiocyanate (FITC) and 7-aminoactinomycin D (7-AAD) (Biolegend), and analyzed by flow cytometry within 1 hour.
Macrophage-mediated platelet phagocytosis
Murine macrophages (RAW 264.7 ATCC) were cultured in 24 wells at 106 per well. Platelets were first incubated with 9D2 and then labeled with FITC-conjugated anti-mouse immunoglobulin (Ig)G. Labeled platelets (107) were added into each well and cocultured with macrophages. For some wells, purified CD8+ T cells were added. After 24 hours, cells were collected, and macrophage-mediated phagocytosis of platelets was assessed by flow cytometry.
In vitro cell proliferation and cytokine production
Splenocytes were incubated with purified CD8+ T cells in a humidified incubator (5% CO2, 3 days, 37°C). For lymphocyte proliferation assays, 5-bromo-2′-deoxyuridine (BrdU; BD Biosciences) was added to the splenocytes (10 μM) during the last 18 hours. Splenocytes were collected and stained with FITC-conjugated anti-mouse CD4 and allophycocyanin (APC)-conjugated anti-mouse CD19 antibodies (Biolegend). Cells were fixed, permeabilized, treated with DNase to expose incorporated BrdU (1 hour, 37°C), and then incubated with PE-conjugated anti-BrdU antibody (5 μL, 20 minutes, 22°C). BrdU content was measured by flow cytometry within 1 hour. Coculture supernatants from splenocytes were assayed using an enzyme-linked immunosorbent assay kit (eBioscience), according to the manufacturer’s instructions to detect interleukin (IL)-10 and other cytokines.
Platelet-associated IgG production was determined with staining of platelets from the coculture supernatant with FITC-anti-mouse IgG and enumerated by flow cytometry as previously described.52
Statistical analysis
Data were analyzed using a Student unpaired t test or 1- or 2-way analysis of variance with Bonferroni posttests, as appropriate. P < .05 was considered significant. Data are presented as mean ± standard error of the mean (SEM) or standard deviation (SD) as indicated. Sample size ranged from 3 to 22, as indicated.
Results
Establishment of murine models of steroid treatment of ITP
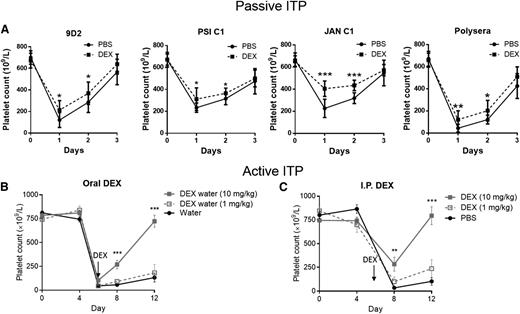
Steroid therapy is the most common first-line treatment of ITP. However, its exact mechanisms of action have not been adequately elucidated. We first developed a panel of mouse anti-mouse mAbs against αIIbβ3 (Table 1), which induces thrombocytopenia when injected into mice.46,47 We then established a passive ITP model using these mAbs to evaluate the efficacy of steroid treatment. We found that daily injections of DEX alone (10 mg/kg, a dose comparable to that used in human treatment of ITP)53,54 does not affect platelet count within 3 to 4 days (supplemental Figure 1, available on the Blood Web site), but administration of DEX at 4 hours after intraperitoneal injection of anti-β3 antisera or mAbs (9D2, PSI C1, or JAN C1) and daily thereafter successfully ameliorated anti-β3 antibody-mediated thrombocytopenia (Figure 1A).
Establishment of murine models of steroid therapy in ITP. (A) The passive model of ITP was generated by injecting anti-β3 integrin polysera or mAbs (9D2, PSI C1, or JAN C1) into WT BALB/c mice. Administration of DEX (10 mg/kg intraperitoneally) 4 hours after mAb injection and continuing daily increased platelet number significantly compared with PBS-treated mice. Mean ± SD. N = 9. (B-C) The active model of ITP was generated in WT mice via engraftment of immunized β3−/− splenocytes (B, 2.5×106; C, 1×106). Administration of DEX (B) orally (N = 22, beginning on day 6) or (C) intraperitoneally (N = 13, beginning at day 6) significantly increased platelet count. *P < .05, **P < .01, ***P < .001 vs water control or PBS. Mean ± SD.
Establishment of murine models of steroid therapy in ITP. (A) The passive model of ITP was generated by injecting anti-β3 integrin polysera or mAbs (9D2, PSI C1, or JAN C1) into WT BALB/c mice. Administration of DEX (10 mg/kg intraperitoneally) 4 hours after mAb injection and continuing daily increased platelet number significantly compared with PBS-treated mice. Mean ± SD. N = 9. (B-C) The active model of ITP was generated in WT mice via engraftment of immunized β3−/− splenocytes (B, 2.5×106; C, 1×106). Administration of DEX (B) orally (N = 22, beginning on day 6) or (C) intraperitoneally (N = 13, beginning at day 6) significantly increased platelet count. *P < .05, **P < .01, ***P < .001 vs water control or PBS. Mean ± SD.
We then transfused splenocytes from WT platelet immunized β3−/− mice to establish the active model of ITP20 and tested steroid therapy. Administration of DEX at day 6, when platelet counts were at their lowest (<100 × 106/mL), successfully reversed thrombocytopenia and elevated platelet counts to >500 × 106/mL by day 12 (Figure 1B-C). Furthermore, intraperitoneal and oral administrations of DEX were shown to have equal efficacy. Cytokine analysis in both active and passive models revealed a similar Th1 profile to that seen in ITP patients,55,56 including increased interferon γ and IL-2, which were normalized after DEX treatment (supplemental Table 1). These are the first reported animal models (ie, EIT) of steroid treatment in ITP that encompasses both the innate and adaptive immune response targeting the major platelet autoantigen (ie, GPIIbIIIa).
CD8+ T-cell depletion enhanced the severity of thrombocytopenia and impaired the response to steroid therapy in the active ITP model
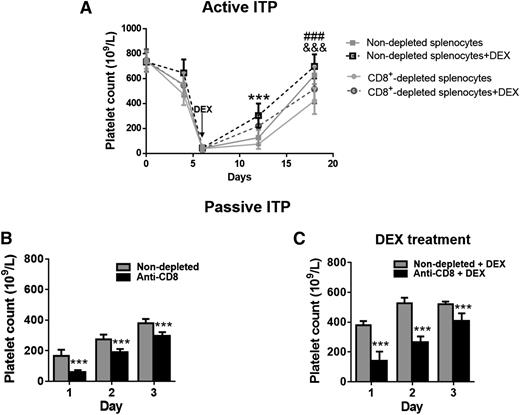
To further elucidate the cellular mechanisms of steroid treatment, we used the above active EIT model20 to study the contributions of individual T-cell populations. It has been well reported that CD8+ CTLs play a pathogenic role in ITP17,18,20 ; therefore, depletion of CD8+ T-cell populations, which would eliminate CTLs, would be expected to attenuate thrombocytopenia and/or enhance the response to therapy. Unexpectedly, in our active EIT model, we found depletion of CD8+ T cells before splenocyte engraftment actually resulted in a more severe thrombocytopenia (Figure 2A), similar to what was observed in a previous report.20 In addition, thrombocytopenia mediated by CD8+-depleted splenocytes was less responsive to DEX treatment compared with thrombocytopenia caused by nondepleted splenocyte engraftment (Figure 2A). These findings demonstrate that, in addition to the previously established cytotoxic function of CD8+ T cells, the CD8+ T-cell population may play a predominantly protective/regulatory role in the context of immune thrombocytopenia, although further investigation in human patients is required.
Depletion of CD8+ T cells results in more severe thrombocytopenia and impairs responsiveness to steroid therapy in vivo. For the active model, WT mice were transplanted with immunized β3−/− splenocytes with or without depletion of CD8+ T cells. DEX treatment began at day 6. (A) Thrombocytopenia was more severe in mice given CD8+ T cell–depleted splenocytes compared with those transplanted with nondepleted splenocytes as indicated by &&&P < .001. Mice transplanted with CD8+ T cell–depleted immunized β3−/− splenocytes were less responsive to oral DEX compared with mice transplanted with nondepleted splenocytes as indicated by ###P < .001. ***P < .001, nondepleted splenocytes vs nondepleted splenocytes + DEX. N = 8. In our passive mouse model, CD8+ T cells were depleted from WT mice by injection of anti-CD8 mAb (400 μg intravenously) before inducing passive ITP with anti-β3 mAb (9D2, 1 μg intraperitoneally). Platelet counts were not significantly affected by anti-CD8 mAb depletion of CD8+ T cells. DEX was administered (10 mg/kg/day, intraperitoneally, daily) beginning at 4 hours after anti-β3 mAb injection. (B) Thrombocytopenia was more severe in CD8+ T cell–depleted mice compared with those given the anti-β3 integrin mAb alone. N = 6. (C) CD8+ T cell–depleted thrombocytopenic mice were less responsive to DEX compared with mice with normal levels of CD8+ T cells. N = 6. ***P < .001. Mean ± SD.
Depletion of CD8+ T cells results in more severe thrombocytopenia and impairs responsiveness to steroid therapy in vivo. For the active model, WT mice were transplanted with immunized β3−/− splenocytes with or without depletion of CD8+ T cells. DEX treatment began at day 6. (A) Thrombocytopenia was more severe in mice given CD8+ T cell–depleted splenocytes compared with those transplanted with nondepleted splenocytes as indicated by &&&P < .001. Mice transplanted with CD8+ T cell–depleted immunized β3−/− splenocytes were less responsive to oral DEX compared with mice transplanted with nondepleted splenocytes as indicated by ###P < .001. ***P < .001, nondepleted splenocytes vs nondepleted splenocytes + DEX. N = 8. In our passive mouse model, CD8+ T cells were depleted from WT mice by injection of anti-CD8 mAb (400 μg intravenously) before inducing passive ITP with anti-β3 mAb (9D2, 1 μg intraperitoneally). Platelet counts were not significantly affected by anti-CD8 mAb depletion of CD8+ T cells. DEX was administered (10 mg/kg/day, intraperitoneally, daily) beginning at 4 hours after anti-β3 mAb injection. (B) Thrombocytopenia was more severe in CD8+ T cell–depleted mice compared with those given the anti-β3 integrin mAb alone. N = 6. (C) CD8+ T cell–depleted thrombocytopenic mice were less responsive to DEX compared with mice with normal levels of CD8+ T cells. N = 6. ***P < .001. Mean ± SD.
CD8+ T-cell depletion enhanced the severity of thrombocytopenia and impaired the response to steroid therapy in passive ITP model
To further investigate CD8+ T-regulatory functions, we depleted CD8+ T cells before induction of thrombocytopenia in WT mice. We injected anti-CD8 antibody on day 1, and on day 2, anti-β3 mAbs were injected to induce thrombocytopenia. Consistent with our findings in the active EIT model, CD8+-depleted mice presented with a more severe thrombocytopenia than nondepleted mice (Figure 2B) and an impaired response to DEX administration (Figure 2C).
CD8+ T-cell transfusion ameliorated thrombocytopenia and enhanced the response to steroid therapy in passive ITP model
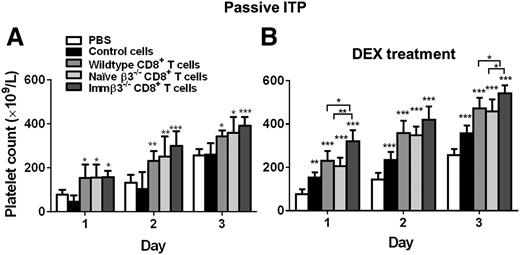
To assess their immunosuppressive role, we transfused purified CD8+ T cells into thrombocytopenic WT mice. We found that transfusion of 106 WT, naïve β3−/−, or immunized β3−/−, CD8+ T cells 4 hours after injection of anti-β3 mAbs all significantly rescued platelet counts compared with controls (Figure 3A). For the control mice, we injected the remaining splenocytes from the CD8+ purification process and found no significant change in platelet count (Figure 3A). This excludes the possibility that attenuated platelet clearance in the other study groups was an artifact due to immune suppression caused by injection of apoptotic cells generated during the purification process.57 Furthermore, transfusion of CD8+ T cells from immunized β3−/− mice appears to be slightly more effective in rescuing platelet counts than the naïve CD8+ T cells (Figure 3A). This suggests that both naïve and antigen-primed CD8+ T cells possess regulatory function. Interestingly, similar to CD4+ Tregs, the CD8+ T-cell immune suppression was not specific to platelet β3 integrin, as CD8+ T cells isolated from splenocytes of β3−/− mice that had been immunized with sheep red blood cells also attenuated anti-β3–mediated platelet clearance (supplemental Figure 2).
CD8+ T-cell transfusion therapeutically attenuated platelet clearance and enhanced response to DEX in passive murine model of ITP. In the passive model of ITP, mice were injected with anti-β3 integrin mAb (9D2, 1 μg, intraperitoneally) at day 0 and transfused with 106 CD8+ T cells from either WT, naïve β3−/−, or immunized β3−/− mice. Control cells are remaining splenocytes following CD8+ purification. (A) CD8+ T cells significantly increased platelet count in the absence of any DEX treatment. N = 6. *P < .05, **P < .01, ***P < .001 vs PBS. (B) DEX (10 mg/kg) was administered at 4 hours after mAb injection and CD8+ T-cell transfusion. N = 6. *P < .05, **P < .01, ***P < .001 vs PBS or indicated groups. Mean ± SD.
CD8+ T-cell transfusion therapeutically attenuated platelet clearance and enhanced response to DEX in passive murine model of ITP. In the passive model of ITP, mice were injected with anti-β3 integrin mAb (9D2, 1 μg, intraperitoneally) at day 0 and transfused with 106 CD8+ T cells from either WT, naïve β3−/−, or immunized β3−/− mice. Control cells are remaining splenocytes following CD8+ purification. (A) CD8+ T cells significantly increased platelet count in the absence of any DEX treatment. N = 6. *P < .05, **P < .01, ***P < .001 vs PBS. (B) DEX (10 mg/kg) was administered at 4 hours after mAb injection and CD8+ T-cell transfusion. N = 6. *P < .05, **P < .01, ***P < .001 vs PBS or indicated groups. Mean ± SD.
Last, we found that transfusion of CD8+ T cells before intraperitoneal administration of DEX resulted in significantly higher platelet counts than administration of DEX alone (Figure 3B). Furthermore, CD8+ T cells from immunized β3−/− mice were significantly more effective in the presence of DEX than were naïve/WT CD8+ T cells. These findings indicate that CD8+ T-cell populations in our EIT models plays a suppressive role, and both naïve and antigen-primed CD8+ T cells possess therapeutic potential, although the antigen-primed CD8+ T cells have greater potency.
Steroid treatment selectively increased CD8+ Tregs in both the passive and active models of ITP
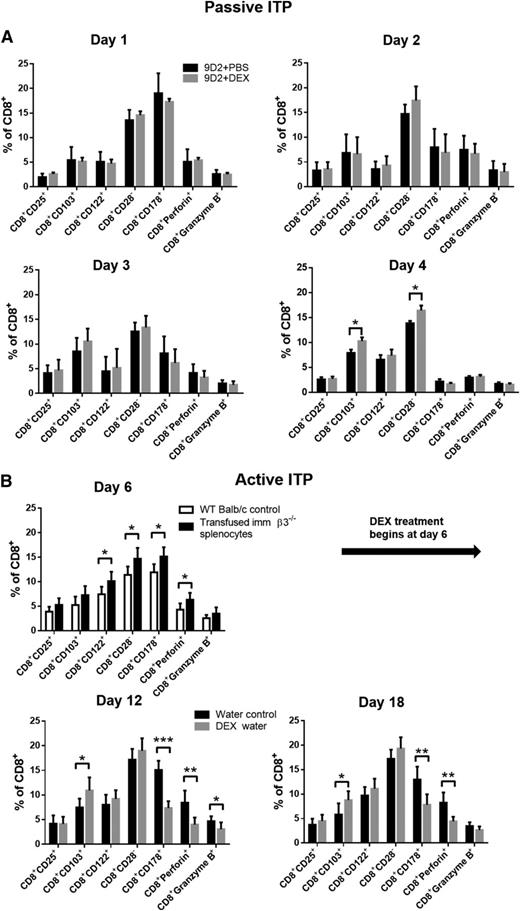
We next evaluated whether some of the previously reported CD8+ Treg subsets58 are present or affected by DEX treatment. In passive EIT, we found DEX injection increased CD8+CD103+ and CD8+CD28− Treg populations (Figure 4A) but had no significant effect on CTL populations (CD8+CD178+, CD8+granzyme B+, and CD8+perforin+). In active EIT, as previously reported,20,59 we found transfusion of immunized β3−/− splenocytes into WT mice resulted in increased populations of CTLs (Figure 4B, day 6), as well as increased CD8+ Treg subsets (ie, before DEX; Figure 4B, day 6). The increase of both engrafted CTLs and CD8+ Tregs is likely due to continued proliferation following antigen stimulation in the WT (β3+/+) recipient mice. Additionally, we found antigen induced CD8+ Treg subsets in the blood, spleen, and thymus, as well as development of memory phenotype after immunization of β3−/− mice with WT platelets (supplemental Figure 3). This antigen-induced CD8+ Treg expansion may account for the increased effectiveness seen with the therapeutic transfusion of immunized β3−/− CD8+ T cells in rescuing platelet counts compared with naïve β3−/− CD8+ T cells (Figure 3). Interestingly, following DEX treatment, we found further increases in CD8+ Treg populations with a concomitant decrease in CTLs, which may partially explain the therapeutic mechanisms of steroid therapy for ITP (Figure 4).
Steroid treatment selectively increased CD8+ Tregs in both the passive and active models of ITP. (A) In the passive model of ITP, mice were injected with anti-β3 mAb (9D2, 1 μg, intraperitoneally) at day 0. In some mice, 10 mg/kg DEX was administered via intraperitoneal injection 4 hours after antibody injection. On indicated days, mice were bled, and different CD8+ subsets were detected via flow cytometry. N = 8 to 10. (B) In the active model, mice were transfused with immunized β3−/− splenocytes on day 0. DEX treatment (oral, 10 mg/kg) or control water was initiated on day 6. Mice were bled on the indicated days, and CD8+ subsets were detected as above. N = 6, *P < .05, **P < .01, ***P < .001. Mean ± SD.
Steroid treatment selectively increased CD8+ Tregs in both the passive and active models of ITP. (A) In the passive model of ITP, mice were injected with anti-β3 mAb (9D2, 1 μg, intraperitoneally) at day 0. In some mice, 10 mg/kg DEX was administered via intraperitoneal injection 4 hours after antibody injection. On indicated days, mice were bled, and different CD8+ subsets were detected via flow cytometry. N = 8 to 10. (B) In the active model, mice were transfused with immunized β3−/− splenocytes on day 0. DEX treatment (oral, 10 mg/kg) or control water was initiated on day 6. Mice were bled on the indicated days, and CD8+ subsets were detected as above. N = 6, *P < .05, **P < .01, ***P < .001. Mean ± SD.
CD8+ T cells suppressed platelet apoptosis and clearance in vitro
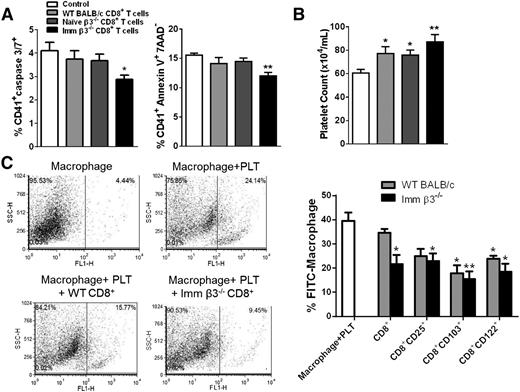
CD8+ CTLs from ITP patients can cause direct platelet lysis, likely through increased expression of granzyme B and perforin.18 However, whether CD8+ Tregs can inhibit platelet apoptosis has not been explored. We thus measured platelet apoptosis and clearance after coculture with CD8+ T cells and CD8+-depleted β3−/− splenocytes in vitro. After a 72-hour incubation, CD8+ T cells from immunized β3−/− mice suppressed platelet apoptosis as measured by caspase-3 and -7 and Annexin-V binding (Figure 5A). In addition, more platelets remained after 72 hours of coculture with β3−/− CD8+ T cells, particularly with the antigen-primed CD8+ T cells (Figure 5B). We further examined whether CD8+ Tregs directly inhibit phagocytosis of opsonized platelets by macrophages, which could contribute to the increased remaining platelets in the coculture system. We found CD8+ Tregs purified from both naïve and immunized β3−/− mice inhibited macrophage phagocytosis (Figure 5C) dose dependently (supplemental Figure 4), although immunized β3−/− CD8+ T cells were more effective. These data are also consistent with the protective role observed in vivo in the passive EIT model (Figure 3).
CD8+ T cells suppressed platelet apoptosis and clearance in vitro. (A-B) CD8+ T cells from immunized β3−/− mice were added to CD8+-depleted splenocytes and then cocultured with WT platelets. Control has no CD8+ T cells added. (A) After 72 hours, platelets remaining in culture were identified by forward and side scatter characteristics and gated on 7-AAD− and assessed for apoptosis with caspase 3/7 and Annexin V. N = 4. (B) CD8+ T cells from immunized β3−/− mice were cocultured with platelets for 72 hours, and then the remaining platelets in solution were counted, with flow cytometry as events per second. More platelets remained in the coculture system after incubation with CD8+ T cells from immunized β3−/− mice compared with control or naïve β3−/− CD8+ T cells. N = 4. (C) Platelets were labeled with 9D2 primary antibody and FITC anti-mouse IgG. Platelets were then cocultured with macrophages with or without CD8+ T cells. After 24 hours, FITC-positive macrophages were detected by flow cytometry. CD8+, CD8+CD25+, CD8+CD122+, and CD8+CD103+ T cells from immunized β3−/− and WT mice significantly suppressed phagocytosis of platelets by macrophages. Furthermore, the CD8+ T cells from immunized β3−/− mice suppressed phagocytosis more effectively (P < .05). This inhibitory function was also dose dependent (supplemental Figure 4) *P < .05, **P < .01. Mean ± SEM.
CD8+ T cells suppressed platelet apoptosis and clearance in vitro. (A-B) CD8+ T cells from immunized β3−/− mice were added to CD8+-depleted splenocytes and then cocultured with WT platelets. Control has no CD8+ T cells added. (A) After 72 hours, platelets remaining in culture were identified by forward and side scatter characteristics and gated on 7-AAD− and assessed for apoptosis with caspase 3/7 and Annexin V. N = 4. (B) CD8+ T cells from immunized β3−/− mice were cocultured with platelets for 72 hours, and then the remaining platelets in solution were counted, with flow cytometry as events per second. More platelets remained in the coculture system after incubation with CD8+ T cells from immunized β3−/− mice compared with control or naïve β3−/− CD8+ T cells. N = 4. (C) Platelets were labeled with 9D2 primary antibody and FITC anti-mouse IgG. Platelets were then cocultured with macrophages with or without CD8+ T cells. After 24 hours, FITC-positive macrophages were detected by flow cytometry. CD8+, CD8+CD25+, CD8+CD122+, and CD8+CD103+ T cells from immunized β3−/− and WT mice significantly suppressed phagocytosis of platelets by macrophages. Furthermore, the CD8+ T cells from immunized β3−/− mice suppressed phagocytosis more effectively (P < .05). This inhibitory function was also dose dependent (supplemental Figure 4) *P < .05, **P < .01. Mean ± SEM.
CD8+ T cells from immunized β3−/− mice inhibit the adaptive anti-platelet immune response in vitro
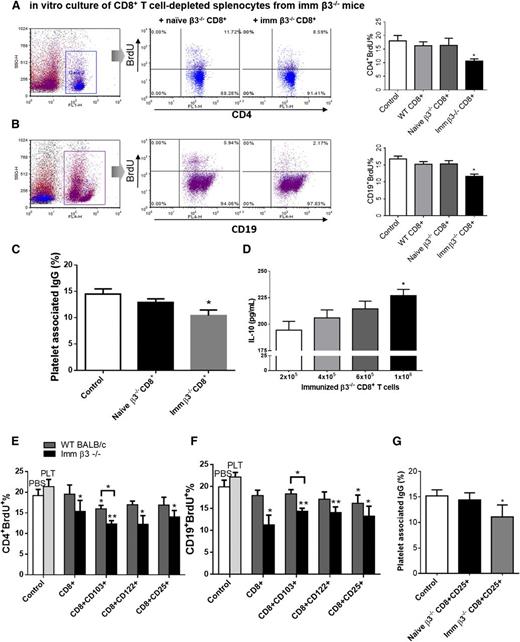
We next investigated CD8+ T-cell regulatory potential against CD4+ T and CD19+ B cells, which are key responders in the propagation of the antiplatelet response through generation of antiplatelet antibodies. We also assessed the effect of CD8+ T cells on their proliferation. CD8+ T cells from the splenocytes of naïve or immunized β3−/− mice were cocultured with immunized CD8+-depleted β3−/− splenocytes for 72 hours in the presence of WT platelets. We found that, although naïve CD8+ T cells showed only weak inhibition (not statistically significant), CD8+ T cells from immunized β3−/− mice significantly inhibited proliferation of CD4+ and CD19+ cells after platelet stimulation (Figure 6A-B) and inhibited CD4+ apoptosis (supplemental Figure 5).
CD8+ Tregs from immunized β3−/− mice inhibit the adaptive antiplatelet immune response in vitro. For proliferation assays, purified splenic CD8+ T cells or CD8+ Tregs from WT, naïve β3−/−, or WT platelet immunized β3−/− mice were added to CD8+ T cell–depleted splenocytes in the presence of 1 × 107 WT platelets per well to induce T- or B-cell proliferation. Control cells were CD8+-depleted splenocytes with PBS or platelets (PLT) as indicated. Both immunized β3−/− CD8+ T cells and CD8+ Tregs inhibited (A,E) CD4+ T-cell proliferation, (B,F) CD19+ B-cell proliferation, and (C,G) platelet-associated IgG production. (D) Immunized β3−/− CD8+ T cells also inhibited IL-10 cytokine production dose dependently. N = 8. Imm, immunized. *P < .05, **P < .01. Mean ± SEM.
CD8+ Tregs from immunized β3−/− mice inhibit the adaptive antiplatelet immune response in vitro. For proliferation assays, purified splenic CD8+ T cells or CD8+ Tregs from WT, naïve β3−/−, or WT platelet immunized β3−/− mice were added to CD8+ T cell–depleted splenocytes in the presence of 1 × 107 WT platelets per well to induce T- or B-cell proliferation. Control cells were CD8+-depleted splenocytes with PBS or platelets (PLT) as indicated. Both immunized β3−/− CD8+ T cells and CD8+ Tregs inhibited (A,E) CD4+ T-cell proliferation, (B,F) CD19+ B-cell proliferation, and (C,G) platelet-associated IgG production. (D) Immunized β3−/− CD8+ T cells also inhibited IL-10 cytokine production dose dependently. N = 8. Imm, immunized. *P < .05, **P < .01. Mean ± SEM.
Concomitantly, immunized β3−/− CD8+ T cells significantly decreased antiplatelet antibody production (Figure 6C). Cytokine analysis revealed increased IL-10 production in the presence of increasing CD8+ T cells in vitro (Figure 6D). Similarly, serum IL-10 levels were increased in immunized β3−/− mice and in mice after DEX treatment in both active and passive models (supplemental Figure 6; supplemental Table 1). These results indicate that CD8+ T cells, especially those antigen-primed, possess regulatory function against CD4+ and CD19+ cells, likely through increased production of IL-10 and possibly also through cell-cell contact.
To determine whether previously detected CD8+ Treg subsets could specifically inhibit the antiplatelet adaptive response, we purified multiple CD8+ Treg subsets from both immunized β3−/− and WT mice, added them to immunized β3−/− CD8+-depleted splenocyte cocultures in the presence of platelets, and measured CD4+ and CD19+ proliferation. These CD8+ Tregs inhibited in vitro proliferation of CD4+ T cells, CD19+ B cells, and antibody generation (Figure 6E-G). Thus, all of these regulatory lineages may contribute to protective functions during ITP, although the implication of these findings requires human studies.
CD8+ Tregs from immunized β3−/− mice inhibit CD8+ cytotoxic activity against platelets
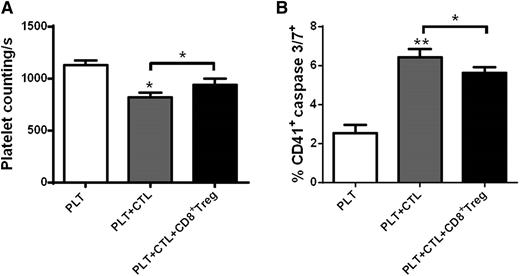
Last, we assessed whether CD8+ Tregs had an inhibitory effect on CD8+ T cell-mediated platelet cytotoxicity. We found, as expected, that in vitro culture of immunized β3−/− CTLs with platelets resulted in increased platelet apoptosis as measured by caspase-3/7. However, addition of immunized β3−/− CD8+ Tregs attenuated CTL-mediated platelet cytotoxicity and increased platelet number (Figure 7). This indicates that CD8+ Tregs have inhibitory potential against CTLs in addition to their effects on CD4+, CD19+, and macrophages and therefore may have important therapeutic potential in ITP.
CD8+ Tregs from immunized β3−/− mice inhibit CD8+ cytotoxic activity against platelets. Platelets (5 × 106) were cultured with purified 1 × 105 cytotoxic CD8+ T cells (CD8+CD178+) with or without 5 × 105 CD8+ Treg cells (according to the original ratio) for 24 hours. Platelet cytotoxicity was measured with (A) remaining platelet number in supernatant, determined as number of platelets per second counted by flow cytometry69 and (B) caspase 3/7 expression. N = 4. PLT, platelet; CTL, cytotoxic CD8+ T cells. *P < .05, **P < .01. Mean ± SEM.
CD8+ Tregs from immunized β3−/− mice inhibit CD8+ cytotoxic activity against platelets. Platelets (5 × 106) were cultured with purified 1 × 105 cytotoxic CD8+ T cells (CD8+CD178+) with or without 5 × 105 CD8+ Treg cells (according to the original ratio) for 24 hours. Platelet cytotoxicity was measured with (A) remaining platelet number in supernatant, determined as number of platelets per second counted by flow cytometry69 and (B) caspase 3/7 expression. N = 4. PLT, platelet; CTL, cytotoxic CD8+ T cells. *P < .05, **P < .01. Mean ± SEM.
Discussion
In this study, we established the first animal models of steroid treatment of ITP, which encapsulate both the humoral (antibody) and cellular immune components (both innate and adaptive response) of the disease. Using these EIT models, we observed that in contrast to their reported pathogenic role, depletion of CD8+ T cells enhanced the severity of thrombocytopenia, and decreased response to DEX. Furthermore, transfusion of CD8+ T cells, particularly those which were antigen-primed, rescued platelet counts in both the active and passive EIT. These data suggest, CD8+ T cells may play an overall regulatory role in the context of immune thrombocytopenia and enhance the therapeutic effect of DEX treatment. Increased numbers of CD8+ Treg subsets were detected after platelet immunization in β3−/− mice and, importantly, after DEX treatment in both active and passive EIT models. In vitro, splenocyte cocultures revealed the regulatory/immunosuppressive functions of CD8+ T cells and its regulatory subsets, including direct inhibition of macrophage phagocytosis, CTL platelet cytotoxicity, CD4+ T-cell and CD19+ B-cell proliferation, and platelet-associated IgG production. Thus, CD8+ Tregs were effective against both antibody and cell-mediated EIT. This was consistent with the increased production of the anti-inflammatory cytokine IL-10. Overall, these findings may switch the prevailing view that CD8+ T cells are predominantly pathogenic in immune thrombocytopenia and may provide insights into the protective roles of CD8+ Tregs, as well as mechanisms of steroid therapy in the disorder, which warrants further studies in human patients.
Recent ITP studies have highlighted that, in addition to autoantibodies, CTLs may contribute to thrombocytopenia via direct cytotoxicity against platelets or megakaryocytes.17-20,59 As was previously reported, depletion of CD19+ B cells before splenocyte engraftment still led to thrombocytopenia,20 suggesting that in the absence of antibodies, CTLs alone can cause ITP and CD8+ Tregs in the engraftments were insufficient to prevent this CTL-mediated thrombocytopenia. However, the precise role of CD8+ T cells in the pathogenesis of ITP within the context of an intact immune response (CD19+ B cells/pathogenic antibodies, CTLs, CD4+ T-helper cells, antigen-presenting cells, etc.) was not previously fully addressed. In our present study, we unexpectedly observed that when CD8+ T cell–depleted splenocytes (lacking both CTLs and CD8+ Tregs) were engrafted into WT mice, the recipients developed more severe thrombocytopenia and were less responsive to DEX treatment compared with mice engrafted with nondepleted splenocytes (Figure 2A). This was contrary to the anticipated outcome that depletion of a pathogenic CD8+ population (ie, CTLs) would result in amelioration of thrombocytopenia and enhanced response to DEX. In addition, transfusion of CD8+ T cells, particularly β3 antigen-primed CD8+ T cells, rescued platelet counts and was able to improve the therapeutic effect of DEX in our passive EIT model. Thus, these results suggest that in the presence of regulatory targets, particularly CD19+ B cells, these CD8+ T cells exert a predominantly protective role, limiting thrombocytopenia caused by antibodies and CTLs and supporting DEX therapy.
The regulatory function of the CD8+ T-cell population was further substantiated by detection of increased subsets of CD8+ Tregs, particularly after DEX treatment. The selective increase in CD8+ Tregs but not CD8+ CTLs after DEX treatment reveals a possible important and previously unidentified target of steroid treatment in ITP, which may be exploited in development of more effective therapies. Our additional finding that CD8+ T cells do support DEX in attenuating the antiplatelet response may be translational in helping identify which ITP patients will benefit from steroid therapy. In particular, patients who respond to steroid therapy may have a larger or increased ratio of a functional CD8+ Treg population, whereas patients who are refractory may exhibit impaired CD8+ Treg response. This concept may apply to both antibody- and cell-mediated (eg, CTLs, low/no detectable autoantibody9,60 ) thrombocytopenia because CD8+ Tregs target both pathways in EIT. A clinical quantitative and functional comparison of CD8+ Tregs between steroid-responsive and nonresponsive patients may prove useful.
In contrast to the CD4+ Tregs (CD4+CD25+FoxP3+), which are more prevalent as a naturally occurring population and have been shown to be decreased in active ITP,24 we found that, in our EIT models, CD8+ Tregs increase in the presence of antigen stimulation (supplemental Figure 3). Inducible CD8+ Tregs have been previously demonstrated in other autoimmune diseases and in chronic infections.35,61 The inducible property of this CD8+ Treg population makes it an attractive therapeutic agent, as autologous CD8+ T cells may be easily expanded ex vivo and reinfused therapeutically.
CD8+ Tregs have been studied in other autoimmune diseases.36,62-64 Several mechanisms have been implicated in the suppressive activity of CD8+ Tregs, including secretion of immunosuppressive cytokines IL-10 and transforming growth factor-β,35 as well as the downregulation of surface CTLA-4.65 Because we observed dose-dependent increases in IL-10 production with increasing CD8+ Tregs and IL-10 has been shown to suppress Th1 polarized responses,66 which is typical of the antiplatelet response in ITP,67 CD8+ Tregs may, through IL-10, exert their protective function in ITP.
In addition to IL-10, contact-dependent mechanisms may also be used by CD8+ Tregs in mediating suppression of both the innate and adaptive immune responses. We found that β3-primed CD8+ T cells possessed stronger inhibitory function against macrophages, CD4+ T cells, and CD19+ B cells than did naïve CD8+ T cells (Figures 5C and 6A-B). This may be due to the activation/proliferation of these CD8+ T cells in our in vitro culture system (containing the same platelet antigen and antigen presenting cells), leading them to exert their inhibitory function with greater potency via cell-cell contact and/or local release of their anti-inflammatory cytokine (eg, IL-10). Notably, although activation and proliferation of Tregs may require antigen stimulation and is antigen specific, the current understanding of immunology is that the effect of Tregs on other cells is not specific,68 which may explain why these naïve naturally occurring (β3−/− and WT; Figures 3 and 5) or other antigen-primed Tregs (eg, sheep red blood cell antigen-induced Tregs; supplemental Figure 2) also ameliorate thrombocytopenia. However, the exact molecular mechanisms of cell-cell contact and cytokine release by CD8+ Tregs, including those naturally occurring (eg, CD8+CD122+) Tregs in ITP, require further investigation.
In summary, we established the first animal models of steroid treatment of ITP that encapsulate both the innate and adaptive immune response of the disease. These EIT models should be valuable in future studies of the mechanisms of steroid therapy and immunopathogenesis of ITP. Furthermore, we demonstrated that CD8+ T cells were required for optimal efficacy of steroid therapy. Our findings revealed a population of CD8+ Tregs that was able to rescue platelet count, suggesting that CD8+ Tregs may impart a protective role that may outweigh the pathogenic function of CD8+ T cells (ie, CTLs) in immune thrombocytopenia. Whether ITP patients with severe thrombocytopenia or are refractory to DEX have a quantitative/functional ratio deficit of CD8+ Tregs, whether these can be used as diagnostic/prognostic biomarkers, and whether ex vivo-expanded CD8+ Tregs have therapeutic potential deserve to be further investigated and confirmed in clinical studies.
Presented in part at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 2013 (poster), and the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 8, 2014 (oral).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr John W. Semple for valuable contributions to data interpretation and manuscript preparation; Dr Yiming Wang and Darko Zdravic for assistance in statistical analysis; and Alexandra H. Marshall for editing during preparation of the manuscript.
This work was supported by Canadian Institutes of Health Research grant MOP 97918; Equipment Funds from the Canada Foundation for Innovation, Canadian Blood Services, and St. Michael’s Hospital. L.M. and I.Y. are recipients of the Postdoctoral Fellowship from Canadian Blood Services. E.S. is a recipient of the Heart and Stroke Foundation of Canada/Ontario Graduate Scholarship in Science & Technology. J.L. is a recipient of the Graduate Fellowship award from the Department of Laboratory Medicine and Pathobiology, University of Toronto. M. Xuan is a recipient of State Scholarship Fund from China Scholarship Council. Y.S. is a recipient of the Development Fund for Young Physicians from the Chinese Physicians’ Association of Hematologic Malignancies. M. Xu is a recipient of State Scholarship Fund from the China Scholarship Council and Ontario Trillium Scholarship, Canada.
Authorship
Contribution: L.M. and J.L. designed the study, performed experiments, and wrote the manuscript; E.S. did the pioneer study; G.Z., P.C., and M.Xuan contributed to study design, experiments, and data analysis; M.Xu, L.B., X.W., and I.Y. performed experiments; Y.S., G.J.P., A.H.L., and J.F. contributed to study design and data interpretation; and H.N. is the principal investigator who designed the study, interpreted results, and prepared the manuscript.
Conflict-of-interest disclosure: H.N. and G.Z. hold a patent (US Patent Application no. 12/082 686; Canadian Patent Application no. 2 628 900; and European Patent Application no. 08153880.3) for the mouse anti-mouse mAbs used in this study. The remaining authors declare no competing financial interests.
Correspondence: Heyu Ni, St. Michael's Hospital, 30 Bond St, Toronto, ON, M5B 1W8, Canada; e-mail: nih@smh.ca.
References
Author notes
L.M., E.S., and J.L. contributed equally to this work.