Key Points
Reduced prothrombin improves survival and ameliorates inflammation and end-organ damage without spontaneous bleeding in sickle cell mice.
An individual procoagulant, prothrombin, represents a novel therapeutic target that can improve sickle cell disease outcome.
Abstract
Sickle cell disease (SCD) results in vascular occlusions, chronic hemolytic anemia, and cumulative organ damage. A conspicuous feature of SCD is chronic inflammation and coagulation system activation. Thrombin (factor IIa [FIIa]) is both a central protease in hemostasis and a key modifier of inflammatory processes. To explore the hypothesis that reduced prothrombin (factor II [FII]) levels in SCD will limit vaso-occlusion, vasculopathy, and inflammation, we used 2 strategies to suppress FII in SCD mice. Weekly administration of FII antisense oligonucleotide “gapmer” to Berkeley SCD mice to selectively reduce circulating FII levels to ∼10% of normal for 15 weeks significantly diminished early mortality. More comprehensive, long-term comparative studies were done using mice with genetic diminution of circulating FII. Here, cohorts of FIIlox/− mice (constitutively carrying ∼10% normal FII) and FIIWT mice were tracked in parallel for a year following the imposition of SCD via hematopoietic stem cell transplantation. This genetically imposed suppression of FII levels resulted in an impressive reduction in inflammation (reduction in leukocytosis, thrombocytosis, and circulating interleukin-6 levels), reduced endothelial cell dysfunction (reduced endothelial activation and circulating soluble vascular cell adhesion molecule), and a significant improvement in SCD-associated end-organ damage (nephropathy, pulmonary hypertension, pulmonary inflammation, liver function, inflammatory infiltration, and microinfarctions). Notably, all of these benefits were achieved with a relatively modest 1.25-fold increase in prothrombin times, and in the absence of hemorrhagic complications. Taken together, these data establish that prothrombin is a powerful modifier of SCD-induced end-organ damage, and present a novel therapeutic target to ameliorate SCD pathologies.
Introduction
Sickle cell disease (SCD) is a common monogenic disorder that affects millions worldwide and is caused by a mutant β-globin gene. It is characterized by erythrocyte sickling, chronic hemolytic anemia, episodic acute vaso-occlusions, chronic systemic inflammation at baseline, and acute and chronic cumulative organ damage.1-7 Therapeutic options to prevent organ pathologies are limited to chronic transfusions, hydroxyurea, or an allogeneic hematopoietic stem cell transplant (HCT).8-12
A conspicuous feature of SCD is chronic activation of the coagulation system, often characterized by high levels of circulating D-dimer, thrombin-antithrombin (TAT) complexes, prothrombin fragment 1.2, increased tissue factor (TF) expression, and high TF-bearing microparticles.13,14 Sickle cell–induced tissue damage is likely 1 driver of procoagulant activation, but sickle red blood cell (RBC) membrane alterations and phosphatidylserine (PS) exposure may further augment procoagulant function.15,16 Thrombocytosis and platelet activation are also well-recognized features of SCD.4,13,14,16-35 Thrombotic events including pulmonary embolism, deep vein thrombosis, and SCD-related stroke are common.17,36-40 SCD-associated pulmonary hypertension (PHT) is associated with endothelial cell activation (as measured by soluble vascular cell adhesion molecule-1 [sVCAM-1]), which would also support procoagulant activity.14,20,23,41 However, the precise contribution of hemostatic factors to SCD-induced pathobiologies and particularly progressive end-organ damage has not been thoroughly explored.
A linkage between hemostatic system activation and inflammation is firmly established and this linkage was underscored in SCD by 2 recent studies in sickle mice.42,43 TF activity on endothelial cells was reported to support increased levels of interleukin-6 (IL-6) in SCD mice, and antibody blockade of TF activity suppressed circulating IL-6, sVCAM-1, and pulmonary neutrophil infiltration.42 Interestingly, rivaroxaban, a small-molecule inhibitor of the prothrombin-activating protease, factor Xa (FXa) decreased IL-6, but not sVCAM-1; dabigatran, a small-molecule inhibitor of thrombin (factor IIa [FIIa]), was reported to not suppress either of these parameters. Thus, TF and FXa, but not FIIa, were linked to endothelial activation and/or inflammatory changes in SCD, and it was proposed that FIIa-independent signaling mechanism(s) mediated inflammatory effects in mice with SCD.43 However, these fascinating studies were short-term in design, and did not explore the influence of hemostatic factors on the wide spectrum of multiorgan pathologies that manifest over long time frames in SCD. Furthermore, human studies exploring the role of coagulation system activation using antithrombotics in SCD patients have primarily focused either on reduction in acute sickle episodes and/or circulating markers of thrombin generation/inflammation as study end points (reviewed in Ataga and Key44 ). The overall results from human studies show that although low-intensity anticoagulation diminished circulating markers of thrombin generation, effects on acute painful episodes were mixed, with either absent, modest, or significant reduction depending on the study.44,45
The central role of FIIa as a hemostatic protease is underscored by the fact that it controls fibrin deposition and stability (through fibrinogen, FXIII, and thrombin activatable fibrinolysis inhibitor), regulates platelet and other cell signaling events (through protease-activated receptors [PARs] PAR-1, -3, and -4) as well as positively and negatively mediates its own generation (through factors XI [FXI] and VIII [FVIII] and protein C). FIIa also constitutes a key regulatory bridge between hemostasis/thrombosis, tissue repair, and inflammatory processes through multiple substrates, including fibrin, PARs, and other effectors.46-49 However, a causative role for FIIa in SCD pathophysiology has remained elusive. We hypothesized that thrombin contributes significantly to the inflammatory changes and vascular occlusions in SCD and, ultimately, worsens the cumulative end-organ damage. To test this concept, we used both pharmacologic and genetic strategies to reduce circulating FII in mice with SCD, such that it only caused mild changes in standard clinical coagulation parameters and no apparent hemorrhagic events. Consequently, a remarkable improvement in inflammation, endothelial dysfunction, multiple SCD-associated organ pathologies and overall survival was observed.
Methods
Experimental mice
Berkeley SCD mice [Tg(Hu-miniLCRα1GγAγδβS) Hba0//Hba0Hbb0//Hbb0 that exclusively express human sickle hemoglobin (HbS)] and C57Bl/6-inbred FIIlox/− mice have been previously described.50,51 Berkeley mice expressing normal human hemoglobin (HbA) were generously provided by Dr Cheryl Hillery (Medical College Wisconsin, Milwaukee, WI). All animals enrolled in these studies were of mixed sex and with generally similar proportions of males and females in each experimental arm. In accordance with standard institutional animal care and use committee practices, Kaplan-Meier studies did not assume death as a primary end point. However, in Berkeley sickle mice, death was rarely preceded by morbidity based on overt health status and behavior, and, therefore, death in individual mice was generally unanticipated and occurred without animals appearing distressed or moribund. Mouse breeding and experiments were conducted in the Cincinnati Children’s Research Foundation Vivarium with appropriate institutional animal care and use committee (IACUC) approvals.
FII-ASO gapmers
FII–antisense oligonucleotide (ASO) gapmer (ISIS 401025; Isis Pharmaceuticals, Inc) known to selectively reduce circulating FII52 or control gapmer (ISIS 141923) were injected weekly into Berkeley SCD mice at 50 mg/kg beginning at weaning. For further details, see supplemental Methods (available on the Blood Web site).
Generation of FII-deficient chimeric sickle mice
Bone marrow cells from Berkeley mice expressing HbS or HbA were transplanted IV into lethally irradiated FIIlox/− or FIIWT recipients in a 1 donor-to-7 recipient ratio to generate HbS/FIIlox/−, HbS/FIIWT, HbA/FIIlox/−, HbA/FIIWT chimeras. Donor chimerism was assessed at 4 months via high-performance liquid chromatography (HPLC) analysis of hemoglobin (Alliance 2960; Waters) using a Poly-CAT column, as previously described.53
Hematologic assessment
Complete blood count was determined on the Hemavet analyzer and reticulocyte count by flow cytometry using Retic-Count reagent (BD Biosciences).
Plasma analysis
Platelet-poor plasma from citrated blood after inferior vena cava phlebotomy was used for determining prothrombin time (PT), D-dimer, TAT complexes, sVCAM-1, and IL-6.
Urine albumin and osmolality
Twenty-four-hour urine collections were used for determining albumin and creatinine (using ELISA kits from Bethyl Laboratories and R&D Systems) and osmolality (using a vapor pressure osmometer Vapro5600 [Wescor Biomedical Systems]).
Histopathology analysis
Mice were weighed before being euthanized. The heart was dissected to separate the right ventricle (RV)-free wall, and left ventricle (LV) + septum was weighed separately. All organs were fixed in 10% buffered formalin and embedded in paraffin. Sections (3-5 µm) were prepared for microscopic evaluation by a pathologist blinded to animal genotype following hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) staining. Lungs were inflation fixed with 4% paraformaldehyde solution in phosphate-buffered saline.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections of lung stained with anti-α-smooth muscle actin (α-SMA) antibody (Sigma-Aldrich) and secondary goat anti-mouse Alexa-Fluor-488 antibody were used for immunohistochemistry; 4′,6′diamino-2-phenylindole solution was added for nuclear staining (described in detail in supplemental Methods). Liver sections were stained for endothelial cells with rabbit anti-CD31 antibody (1:50 dilution; Abcam), followed by incubation with species-specific secondary antibodies, and detected with the UltraMax DAB kit (Ventana Laboratories).
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging (CMR) was performed as previously described.54
Statistical analysis
Data were analyzed using Graphpad Prism version 6. Values are expressed as mean ± standard error of the mean (SEM). The primary comparison of interest for sickle and normal genotypes, separately, was between normal or low prothrombin mice, hence the use of pairwise comparisons. Nonparametric Mann-Whitney U tests were used unless otherwise indicated.
Results
FII-specific ASO decreases early mortality in Berkeley sickle mice
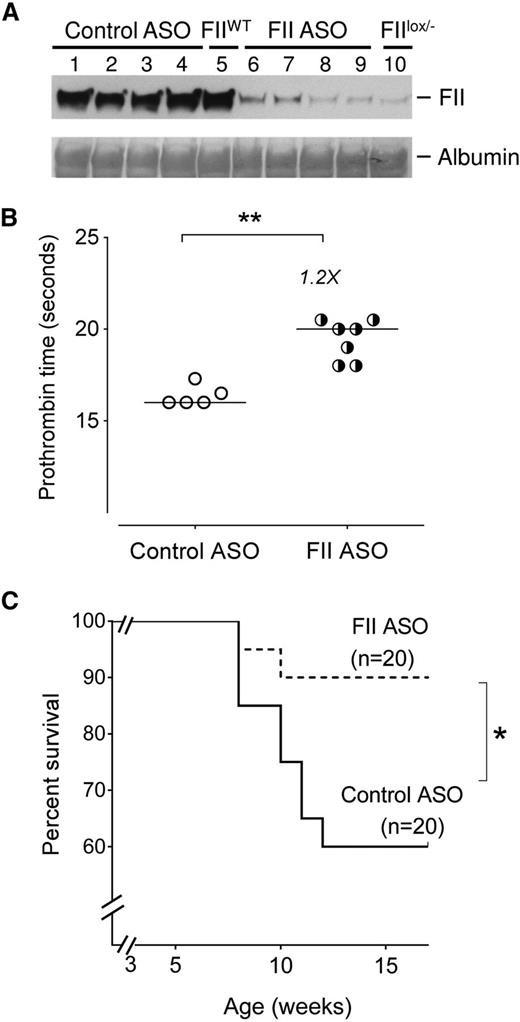
As an initial approach, to explore the impact of prothrombin on SCD outcome, we tested the hypothesis that reducing factor II (FII) levels improves SCD survival. Our Berkeley sickle mouse colony has a relatively high early mortality, with 40% of SCD mice dying within 4 to 5 months (supplemental Figure 1). Cohorts of Berkeley sickle mice were injected with either a murine FII-specific or a control ASO gapmer starting at 3 weeks of age for up to 15 weeks of age (supplemental Figure 3A). The FII-specific ASO gapmer is complementary to the 3′ noncoding portion of the F2 messenger RNA (mRNA, a sequence found to be unique in the mouse genome),52,55 and specifically reduces hepatic F2 mRNA (thereby reducing circulating FII levels) without any impact on other coagulation system components (supplemental Figure 2). The FII-specific ASO dose used reduced circulating FII levels to ∼10% of normal (Figure 1A), a level of FII found here (Figure 1B) and previously55,56 to prolong PTs mildly without causing spontaneous bleeding. More importantly, sickle mice treated with FII ASO showed significantly improved survival compared with sickle mice administered control ASO (Figure 1C). Indeed, the survival of Berkeley SCD mice treated with control ASO did not differ from the untreated Berkeley sickle mouse colony (supplemental Figure 1). These data show that low levels of FII exert a major survival advantage to sickle mice, conceivably reducing the general severity of SCD complications.
Reduction of circulating FII using ASO reduces early mortality in Berkeley sickle mice. (A) Western blot analysis showing levels of circulating FII protein in representative Berkeley sickle mice administered either control ASO (n = 4, lanes 1-4) or FII-specific ASO (n = 4, lanes 6-9) beginning at 3 weeks of age and sacrificed for FII analysis at 15 weeks of age. The plasma FII levels in representative untreated FIIWT (lane 5) and untreated FIIlox/− (lane 10) mice are also shown as additional controls for comparison. (B) PTs of Berkeley sickle mice administered control ASO or FII ASO beginning at 3 weeks of age and sacrificed for plasma analysis at 15 weeks of age. Each symbol represents an individual animal and the bar indicates the median values. Data were analyzed by Mann-Whitney U test; **P < .01. (C) Kaplan-Meier survival analysis of cohorts of Berkeley sickle mice administered either FII ASO (n = 20) or control ASO (n = 20) beginning at 3 weeks of age and continued on weekly ASO treatments until 15 weeks of age. Equal numbers of males and females were enrolled for either control-ASO or FII-specific ASO administration. Among the sickle mice that had early mortalities, the ratio of males to females was 1:1. Comparison of survival curves using log-rank (Mantel-Cox) test; *P < .05.
Reduction of circulating FII using ASO reduces early mortality in Berkeley sickle mice. (A) Western blot analysis showing levels of circulating FII protein in representative Berkeley sickle mice administered either control ASO (n = 4, lanes 1-4) or FII-specific ASO (n = 4, lanes 6-9) beginning at 3 weeks of age and sacrificed for FII analysis at 15 weeks of age. The plasma FII levels in representative untreated FIIWT (lane 5) and untreated FIIlox/− (lane 10) mice are also shown as additional controls for comparison. (B) PTs of Berkeley sickle mice administered control ASO or FII ASO beginning at 3 weeks of age and sacrificed for plasma analysis at 15 weeks of age. Each symbol represents an individual animal and the bar indicates the median values. Data were analyzed by Mann-Whitney U test; **P < .01. (C) Kaplan-Meier survival analysis of cohorts of Berkeley sickle mice administered either FII ASO (n = 20) or control ASO (n = 20) beginning at 3 weeks of age and continued on weekly ASO treatments until 15 weeks of age. Equal numbers of males and females were enrolled for either control-ASO or FII-specific ASO administration. Among the sickle mice that had early mortalities, the ratio of males to females was 1:1. Comparison of survival curves using log-rank (Mantel-Cox) test; *P < .05.
Although this study was primarily designed to be a survival analysis, we attempted to analyze differences in the surviving mice, with the caveat that we were comparing “surviving fit” control-ASO mice with FII-ASO mice. The body weights were not significantly different between control-ASO or FII-ASO–treated sickle mice (supplemental Figure 3B). PTs were modestly but significantly (1.2-fold) higher in FII-ASO mice compared with controls (Figure 1B). D-dimer levels were reduced to 371 ± 48 ng/mL in the FII-ASO group as compared with 468 ± 69 in control-ASO mice, although these differences were not statistically significant. TAT complexes (which are short-lived, and only reflect any proximal/short-term thrombin generated, as opposed to the sum of episodic thrombin generation over long time frames [as likely in SCD]) were not different in either group of sickle mice surviving at 15 weeks of age.
In addition, we attempted complementary studies using separate cohorts of sickle mice randomized at 3 weeks of age to FII-specific or control ASO, that were sacrificed at 9 weeks of age, the mid time point where significant early mortalities occur in sickle mice to assess differences in organ pathology (the experimental schema is shown in supplemental Figure 4A). All sickle mice exhibited some organ pathology. However, at this early age, no major pathological differences in kidney, lung, liver, heart, and other tissues other than vascular congestion were seen between control and FII-ASO mice (supplemental Figure 4B), suggesting that the survival benefits conferred by FII-ASO gapmer in young SCD mice may be due to the evasion of an acute sickle event rather than cumulative end-organ damage. Although the precise cause(s) of death in very young sickle cell mice was not obvious based on histologic tissue surveys, and remains to be formally established, reduced prothrombin presented a clear benefit in life expectancy.
Genetically imposed reduction in FII levels in SCD mice reduces thrombin generation
To gain better functional insight into the long-term advantages of diminished FII levels in sickle mice, and to do so without potential animal stress or challenges associated with weekly ASO-gapmer administration, we used a FII germline mutation approach. Here, using HCT, we generated sickle hematopoietic chimeras on the FIIlox/− background to derive SCD mice carrying ∼10% of the normal level of FII (HbS/FIIlox/−). This approach allowed for a fixed diminution of FII from the time FIIlox/− mice develop SCD through the entire study period (∼1 year following HCT). Control mice were sickle chimeras with normal FII levels (wild-type [WT] mice transplanted with Berkeley sickle bone marrow; HbS/FIIWT). Two additional controls were FIIWT or FIIlox/− mice that received bone marrow transplant from Berkeley donors that express normal human hemoglobin (HbA), to generate HbA/FIIWT and HbA/FIIlox/− chimeras. The experimental schema for these experiments, with the different analysis performed and timelines, is shown in supplemental Figure 5. Only mice that were fully chimeric for donor HbS or HbA and had negligible mouse hemoglobin (<0.3%, as shown in supplemental Figure 6) were studied.
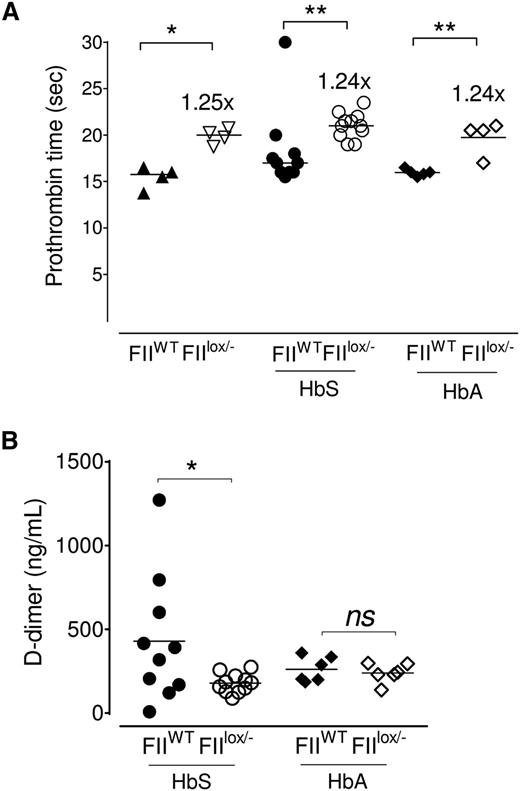
HbS/FIIlox/− and HbA/FIIlox/− chimeric mice had a mild (1.25-fold) increase in PT (Figure 2A) in comparison with HbS/FIIWT or HbA/FIIWT chimeric animals. This is a similar difference in PT as was described in the original description of the FIIlox/− mice50 and to the PT achieved in the FII-ASO–treated mice (Figure 1B). Predictably, in the HbA/FIIlox/− chimeras, both TAT and D-dimer were significantly low, when compared with HbA/FIIWT mice: D-dimer levels were reduced in HbS/FIIlox/− mice relative to FII-sufficient sickle (HbS/FIIWT) mice, consistent with decreased baseline thrombin generation (Figure 2B); and, as would be expected with a diminution in baseline FII, there was an approximately twofold reduction in TAT complexes in HbS/FIIlox/− mice vs HbS/FIIWT mice (supplemental Figure 7). In HbA/FIIWT mice, TAT levels were variable, though generally quite low. When compared with HbA/FIIWT mice, only a fraction of HbS/FIIWT mice exhibited high TAT levels, possibly reflecting episodic sickle cell–associated thrombin generation events. As a result, the average TAT levels of HbS/FIIWT animals were not statistically different from the HbA/FIIWT mice (supplemental Figure 7). TAT levels in nontransplanted Berkeley sickle mice also showed similar patterns, with some mice showing high TAT levels and others not, and were also not significantly different compared with Berkeley HbA animals (data not shown).
Sickle chimeras with 10% circulating FII exhibit a modest increase in PT and diminished evidence of coagulation activation. (A) PT values in FIIWT mice (with 100% circulating FII) and FIIlox/− mice (with ∼10% FII levels), and cohorts of FIIWT and FIIlox/− mice transplanted with either bone marrow from donor Berkeley mice expressing HbA (HbA/FIIWT and HbA/FIIlox/−) or donor Berkeley sickle mice expressing HbS (HbS/FIIWT and HbS/FIIlox/−). (B) D-dimer levels in HbS/FIIWT, HbS/FIIlox/−, HbA/FIIWT, and HbA/FIIlox/− mice. The bar indicates the median. Each symbol represents an individual animal. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year after transplantation. Cohorts were analyzed by Mann-Whitney U test and statistical significance between FIIWT and FIIlox/− chimeras is indicated by asterisks: **P < .01, *P < .05.
Sickle chimeras with 10% circulating FII exhibit a modest increase in PT and diminished evidence of coagulation activation. (A) PT values in FIIWT mice (with 100% circulating FII) and FIIlox/− mice (with ∼10% FII levels), and cohorts of FIIWT and FIIlox/− mice transplanted with either bone marrow from donor Berkeley mice expressing HbA (HbA/FIIWT and HbA/FIIlox/−) or donor Berkeley sickle mice expressing HbS (HbS/FIIWT and HbS/FIIlox/−). (B) D-dimer levels in HbS/FIIWT, HbS/FIIlox/−, HbA/FIIWT, and HbA/FIIlox/− mice. The bar indicates the median. Each symbol represents an individual animal. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year after transplantation. Cohorts were analyzed by Mann-Whitney U test and statistical significance between FIIWT and FIIlox/− chimeras is indicated by asterisks: **P < .01, *P < .05.
Reduction in FII levels in SCD decreases inflammation and endothelial cell dysfunction
As expected, diminution of FII did not alter the RBC parameters between HbS/FIIWT and HbS/FIIlox/− mice, with both HbS chimeras showing RBC features consistent with classic sickle cell anemia. RBC parameters were normal in HbA/FIIWT and HbA/FIIlox/− mice (supplemental Table 1). However, HbS/FIIlox/− mice had significant reduction in the SCD-associated leukocytosis (specifically neutrophilia and monocytosis) relative to HbS/FIIWT chimeras. Furthermore, the SCD-associated thrombocytosis, present in the HbS/FIIWT mice was also significantly diminished in the HbS/FIIlox/− animals (Figure 3A-D). As anticipated, no change in the otherwise normal leukocyte, neutrophil, or platelet counts was seen in HbA/FIIWT vs HbA/FIIlox/− animals, although monocyte numbers were lower in HbA/FIIlox/− mice. There was no significant difference in the lymphocyte count between these animals (supplemental Figure 8).
Sickle chimeras with genetically imposed reductions in circulating FII exhibit significant decreases in inflammation. Total (A) WBC, (B) neutrophil, (C) monocyte, and (D) platelet counts in HbS/FIIWT, HbS/FIIlox/−, HbA/FIIWT, and HbA/FIIlox/− mice are shown. (E) Plasma IL-6 and (F) plasma sVCAM-1 levels in HbS/FIIWT, HbS/FIIlox/−, HbA/FIIWT, and HbA/FIIlox/− cohorts. Each symbol represents an individual animal. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year posttransplantation. sVCAM-1 levels in female mice trended to be lower compared with males within each group. In male mice, sVCAM-1 levels HbS/FIIWT vs HbS/FIIlox/− mice were 17 965 ± 3406 ng/mL vs 12 870 ± 338 ng/mL, respectively. In female mice, sVCAM-1 levels in HbS/FIIWT vs HbS/FIIlox/− mice were 13 009 ± 1361 ng/mL vs 10 914 ± 937 ng/mL, respectively. The differences between the 2 sexes in the different groups were not statistically significant. Cohorts were analyzed by Mann-Whitney U test and statistical significance between FIIWT and FIIlox/− chimeras is indicated by asterisks: **P < .01, *P < .05, ***P < .001.
Sickle chimeras with genetically imposed reductions in circulating FII exhibit significant decreases in inflammation. Total (A) WBC, (B) neutrophil, (C) monocyte, and (D) platelet counts in HbS/FIIWT, HbS/FIIlox/−, HbA/FIIWT, and HbA/FIIlox/− mice are shown. (E) Plasma IL-6 and (F) plasma sVCAM-1 levels in HbS/FIIWT, HbS/FIIlox/−, HbA/FIIWT, and HbA/FIIlox/− cohorts. Each symbol represents an individual animal. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year posttransplantation. sVCAM-1 levels in female mice trended to be lower compared with males within each group. In male mice, sVCAM-1 levels HbS/FIIWT vs HbS/FIIlox/− mice were 17 965 ± 3406 ng/mL vs 12 870 ± 338 ng/mL, respectively. In female mice, sVCAM-1 levels in HbS/FIIWT vs HbS/FIIlox/− mice were 13 009 ± 1361 ng/mL vs 10 914 ± 937 ng/mL, respectively. The differences between the 2 sexes in the different groups were not statistically significant. Cohorts were analyzed by Mann-Whitney U test and statistical significance between FIIWT and FIIlox/− chimeras is indicated by asterisks: **P < .01, *P < .05, ***P < .001.
This diminution of leukocytosis FII in HbS/FIIlox/− animals was also accompanied by significantly reduced IL-6, a systemic marker of inflammation, and reduced levels of the endothelial cell activation marker sVCAM-1, as compared with HbS/FIIWT mice (Figure 3E-F). These markers of inflammation and endothelial dysfunction were at low levels in HbA/FIIWT mice and remained unchanged in HbA/FIIlox/− animals.
Reduction in FII levels in SCD protects against sickle nephropathy
To assess the impact of lowered FII on the cumulative organ damage associated with SCD, we examined its effect on sickle nephropathy, a serious complication that results in progressive albuminuria in 50% to 70% of adults and renal failure and mortality in ∼30% of SCD patients. The nephropathy of SCD is characterized by both a tubular dysfunction, that manifests early as hyposthenuria, and glomerular damage, which manifests as glomerular hypertrophy, expansion of the mesangium, thickening of capillary loops and glomerular basement membrane, significant albuminuria and focal segmental glomerulosclerosis (FSGS), all of which result in progressive nephron loss and renal insufficiency.57-59 Consistent with the renal histopathological changes in SCD individuals, HbS/FIIWT mice show similar glomerular lesions, and all of these glomerular pathologies were significantly reduced in HbS/FIIlox/− mice (Figure 4A-E). Tubular pathology was also improved in HbS/FIIlox/− mice (Figure 4F). Semiquantitative histological scoring of glomerular, vascular, and tubular changes was performed by the study pathologist who was blinded to the mouse genotypes. We observed a remarkable improvement in the kidney pathology scores (Figure 4G-J). There were minimal glomerular changes in HbA/FIIWT mice, deemed to be secondary to irradiation for HCT, that were also improved in HbA/FIIlox/− mice (supplemental Figure 9).
Sickle chimeras with low FII levels are protected against kidney damage and sickle nephropathy. (A-F) Representative H&E kidney sections comparisons between HbS/FIIWT (left panel) and HbS/FIIlox/− (right panel) mice showing (A) ischemia, inflammatory cell infiltration, and renal scarring (×10 objective). (B-C) FSGS and glomerular atrophy (shown by arrows) in HbS/FIIWT glomeruli compared with HbS/FIIlox/− glomeruli. (D-E) PAS staining showing glomerular basement membrane thickening in HbS/FIIWT glomeruli compared with HbS/FIIlox/− glomeruli. (F) Representative H&E-stained sections of renal tubules showing tubular loss, inflammatory cell infiltration (indicated by dotted lines), and regenerative tubules. (B-F) Objective, ×60. (G-J) Semiquantitative assessment of histologic features. Histology scores ranged from 0 to 5, where 0 represented normal kidney morphology; 1, changes in <15% of glomerular area; 2, 20% to 30% of the glomerular area; 3, 40% to 50% of the glomerular area; 4, ∼60% to 70% of glomerular area; 5, severe changes in most all glomeruli. (K) Urine albumin and (L) urine osmolality values. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year posttransplantation. Each symbol represents an individual animal. Statistical analyses on the histologic scores were done by Mann-Whitney U test. Statistical significance between HbS/FIIWT (n = 6) and HbS/FIIlox/− (n = 7) mice is indicated by asterisks: ****P < .0001, ***P < .01, **P < .01, *P < .05.
Sickle chimeras with low FII levels are protected against kidney damage and sickle nephropathy. (A-F) Representative H&E kidney sections comparisons between HbS/FIIWT (left panel) and HbS/FIIlox/− (right panel) mice showing (A) ischemia, inflammatory cell infiltration, and renal scarring (×10 objective). (B-C) FSGS and glomerular atrophy (shown by arrows) in HbS/FIIWT glomeruli compared with HbS/FIIlox/− glomeruli. (D-E) PAS staining showing glomerular basement membrane thickening in HbS/FIIWT glomeruli compared with HbS/FIIlox/− glomeruli. (F) Representative H&E-stained sections of renal tubules showing tubular loss, inflammatory cell infiltration (indicated by dotted lines), and regenerative tubules. (B-F) Objective, ×60. (G-J) Semiquantitative assessment of histologic features. Histology scores ranged from 0 to 5, where 0 represented normal kidney morphology; 1, changes in <15% of glomerular area; 2, 20% to 30% of the glomerular area; 3, 40% to 50% of the glomerular area; 4, ∼60% to 70% of glomerular area; 5, severe changes in most all glomeruli. (K) Urine albumin and (L) urine osmolality values. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year posttransplantation. Each symbol represents an individual animal. Statistical analyses on the histologic scores were done by Mann-Whitney U test. Statistical significance between HbS/FIIWT (n = 6) and HbS/FIIlox/− (n = 7) mice is indicated by asterisks: ****P < .0001, ***P < .01, **P < .01, *P < .05.
Functionally, HbS/FIIWT mice had hyposthenuria and significant albuminuria (Figure 4K-L). Consistent with the improved histology, HbS/FIIlox/− mice showed a highly significant decrease in albuminuria. Although hyposthenuria was also significantly improved, this difference was not as pronounced as the improvement in albuminuria. These results indicate that procoagulant function significantly contributes to the renal and, specifically, the glomerular pathology in SCD, and lowering FII in mice with SCD protects against development of sickle nephropathy.
Reduction in FII results in improved SCD-associated cardiopulmonary pathologies
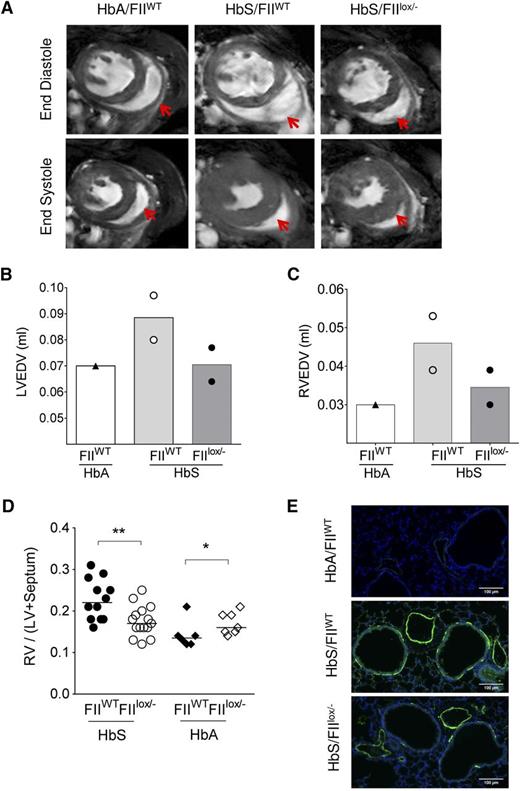
PHT and ventricular diastolic dysfunction are recognized complications of SCD and have been shown to be associated with coagulation activation, endothelial dysfunction, and inflammation.14,34 Although the incidence of right heart catheterization–proven PHT in SCD adults is now estimated at 6% to 10%,60,61 we54 and others62 have commonly observed development of PHT in SCD mice with increasing age. We have previously shown that RV enlargement and associated increased RV mass (as determined by CMR or physical mass) correlates with increased RV pressures on cardiac catheterization in Berkeley SCD mice.54 We therefore assessed the RV in HbS/FIIlox/− and HbS/FIIWT mice. CMR was done in 2 each of HbS/FIIWT and HbS/FIIlox/− mice, and one HbA/FIIWT control. RV wall thickness was increased in HbS/FIIWT mice, as compared with the HbA/FIIWT control (Figure 5A). In addition, RV and LV end-diastolic volumes were increased in the presence of normal ejection fraction, indicative of ventricular dilatation. These features were less pronounced in the HbS/FIIlox/− mice compared with HbS/FIIWT mice, although the animal numbers were limited (Figure 5B-C). As in humans with SCD, systolic function was normal in HbS chimeras (supplemental Figure 10). RV and LV weights confirmed the CMR data in larger numbers of animals, showing a significant decrease in the RV/LV + septum ratio in HbS/FIIlox/− mice as compared with HbS/FIIWT mice (Figure 5D).
Sickle chimeras with genetically reduced FII levels have improved cardiopulmonary pathology and function. (A) CMR on representative HbA/FIIWT, HbS/FIIWT, and HbS/FIIlox/− mice at 1 year following bone marrow transplantation. A representative view of the ventricles is shown with RV in the same plane, which is indicated by the red arrows. (B-C) LV and RV end-diastole volumes in chimeric mice; symbols represent the value of each individual mouse and the bars denote the average. (D) RV hypertrophy is measured as a ratio of RV wall weight/LV + septum weights (Fulton Index) in HbS/FIIWT (n = 12), HbS/FIIlox/− (n = 15), HbA/FIIWT (n = 6), HbA/FIIlox/− (n = 7) mice. Cohorts were analyzed by Mann-Whitney U test. Statistical significance between FIIWT and FIIlox/− chimeras is indicated by asterisks: **P < .01, *P < .05. (E) α-SMA staining in lung tissue. Images are representative of 5 to 7 mice per group. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year after transplantation.
Sickle chimeras with genetically reduced FII levels have improved cardiopulmonary pathology and function. (A) CMR on representative HbA/FIIWT, HbS/FIIWT, and HbS/FIIlox/− mice at 1 year following bone marrow transplantation. A representative view of the ventricles is shown with RV in the same plane, which is indicated by the red arrows. (B-C) LV and RV end-diastole volumes in chimeric mice; symbols represent the value of each individual mouse and the bars denote the average. (D) RV hypertrophy is measured as a ratio of RV wall weight/LV + septum weights (Fulton Index) in HbS/FIIWT (n = 12), HbS/FIIlox/− (n = 15), HbA/FIIWT (n = 6), HbA/FIIlox/− (n = 7) mice. Cohorts were analyzed by Mann-Whitney U test. Statistical significance between FIIWT and FIIlox/− chimeras is indicated by asterisks: **P < .01, *P < .05. (E) α-SMA staining in lung tissue. Images are representative of 5 to 7 mice per group. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year after transplantation.
PHT is also characterized by proliferation of smooth muscles and thickening of tunica media of pulmonary arteries/arterioles. In mice, instead of the classic concentric smooth muscle thickening seen in humans and rats,63 an increased number of cells expressing α-SMA is observed.64 Lung sections of HbS/FIIlox/− mice showed decreased α-SMA staining around the blood vessels as compared with HbS/FIIWT mice (Figure 5E). In addition, increased α-SMA was also seen around the airways in HbS/FIIWT mice relative to HbS/FIIlox/− mice. The latter is a feature of airway reactivity, another known association with SCD.65-67 As anticipated, PHT and other associated pulmonary pathologies were absent in HbA chimeras.
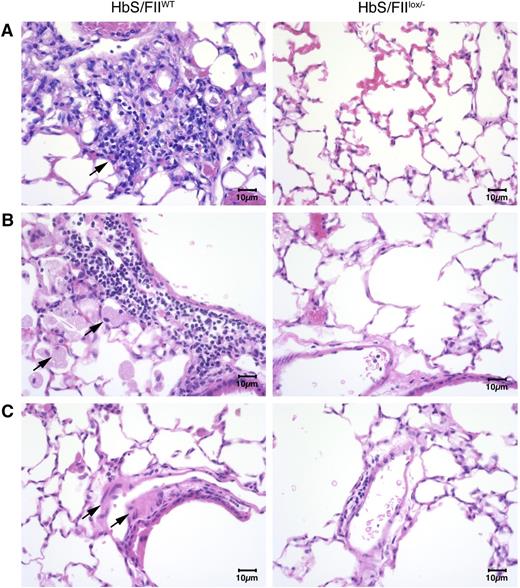
Additionally, lung histology revealed the presence of foamy alveolar macrophages, thickened arterial/arteriolar tunica media, and increased lymphocyte and macrophage infiltration (Figure 6). Edema around the blood vessels and vascular congestion was also pronounced in HbS/FIIWT mice. In contrast, all of these pathologies were remarkably reduced in HbS/FIIlox/−mice (Figure 6). These changes were absent in both of the HbA chimeric controls (supplemental Figure 11).
Sickle chimeras with 10% of normal FII levels have diminished pulmonary pathology. (A-C) Representative lung sections of HbS/FIIWT mice (left panels) compared with that of HbS/FIIlox/− mice (right panels) showing: (A) increased inflammatory infiltrate in HbS/FIIWT lungs compared with HbS/FIIlox/− lungs. Objective, ×10. (B) Increased alveolar macrophages in HbS/FIIWT lungs compared with HbS/FIIlox/− lungs. Objective, ×40. (C) Increased edema around blood vessels in HbS/FIIWT lungs vs its lack in HbS/FIIlox/− lungs. Objective, ×40. Black arrows indicate the pathology described in each panel. Images are representative of 6 to 9 mice per experimental group. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year posttransplantation.
Sickle chimeras with 10% of normal FII levels have diminished pulmonary pathology. (A-C) Representative lung sections of HbS/FIIWT mice (left panels) compared with that of HbS/FIIlox/− mice (right panels) showing: (A) increased inflammatory infiltrate in HbS/FIIWT lungs compared with HbS/FIIlox/− lungs. Objective, ×10. (B) Increased alveolar macrophages in HbS/FIIWT lungs compared with HbS/FIIlox/− lungs. Objective, ×40. (C) Increased edema around blood vessels in HbS/FIIWT lungs vs its lack in HbS/FIIlox/− lungs. Objective, ×40. Black arrows indicate the pathology described in each panel. Images are representative of 6 to 9 mice per experimental group. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year posttransplantation.
Together, these data indicate that increased thrombin generation contributes to the cardiopulmonary pathologies in SCD; and consistent with a decrease in circulatory markers of inflammation, pulmonary inflammation is also reduced with diminution of circulating FII.
Reduced FII levels protect against hepatic inflammation and coagulative necrosis
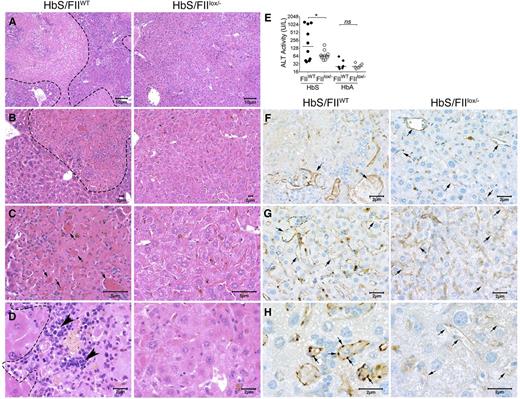
The hepatic pathophysiology in SCD results from ischemia, vascular occlusion, and acute hepatic sequestration. In HbS/FIIWT mice, multifocal areas of coagulative hepatic necrosis (Figure 7A-D) were observed. Necrotic areas were infiltrated with lymphocytes and macrophages in their periphery, and marked congestion within hepatic sinusoids with shrinkage of hepatocytes were observed (Figure 7A-D). Examination of the hepatic vasculature revealed massively engorged blood vessels filled with sickle RBCs and likely represents sludging of the blood flow in the liver vasculature. In some areas, these vascular engorgements were associated with accompanying necrosis, suggesting vascular blockade (Figure 7B-C,F-H). These occlusive events were significantly reduced in HbS/FIIlox/− mice. Of note, there was no difference in microvascular density between HbS/FIIlox/− and HbS/FIIWT mice. Indeed, in HbS/FIIlox/− mice, areas of focal hepatic necrosis were fewer and more limited, and completely absent in a few animals. Additionally, reduced hepatic inflammatory infiltrates were seen. We also stained liver sections with CD31, an endothelial cell marker, and show that additionally, vessels were congested with reactive endothelium in HbS/FIIWT liver sections, which was not a prominent feature in HbS/FIIlox/− liver sections (Figure 7F-H). Consistent with the diminished microscopic evidence of hepatic damage, plasma alanine transaminase activity was also significantly lower in HbS/FIIlox/− mice compared with HbS/FIIWT mice (Figure 7E). No hepatic histopathological changes or elevation in transaminase activity was observed in HbA chimeras, regardless of FII levels (Figure 7E; supplemental Figure 12).
Sickle chimeras with genetically reduced FII levels have improved hepatic morphology, function, and reduced vascular occlusions. Representative H&E liver sections from HbS/FIIWT (left panel, A-D) and HbS/FIIlox/− (right panel, A-D) mice 1 year following marrow transplant viewed at various magnifications (objectives: A, ×10; B, ×20; C, ×40; and D, ×60) showing multifocal areas of coagulative hepatic necrosis with occasionally associated massively congested blood vessels (shown by arrows in panel C) in HbS/FIIWT mice. Areas of necrosis are marked by dotted lines (A-B,D) and infiltrating leukocytes are marked by arrowheads (D) in HbS/FIIWT mice. In HbS/FIIlox/− mice, the areas of coagulative necrosis were minimal with comparatively fewer congested blood vessels seen. (F-H) CD31 staining of liver vascular endothelial cells showing vascular congestion in HbS/FIIWT mice (shown by arrows) along with prominent rounded endothelial cells (H). These changes were minimal in HbS/FIIlox/− mice. No differences were observed in the microvascular density (MVD) in liver sections from HbA/FIIWT and HbA/FIIlox/− mice. (F-G) Objective, ×40. (H) Objective, ×100. Images are representative of 9 mice per experimental group. (E) Alanine transaminase (ALT) activity in the serum of HbS/FIIWT, HbS/FIIlox/−, HbA/FIIWT, and HbS/FIIlox/− mice. Each symbol represents an individual animal and lines represent median values. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year after transplantation. Cohorts were analyzed by Mann-Whitney U test and statistical significance between FIIWT and FIIlox/− chimeras is indicated by asterisks: *P < .05. n.s., not significant.
Sickle chimeras with genetically reduced FII levels have improved hepatic morphology, function, and reduced vascular occlusions. Representative H&E liver sections from HbS/FIIWT (left panel, A-D) and HbS/FIIlox/− (right panel, A-D) mice 1 year following marrow transplant viewed at various magnifications (objectives: A, ×10; B, ×20; C, ×40; and D, ×60) showing multifocal areas of coagulative hepatic necrosis with occasionally associated massively congested blood vessels (shown by arrows in panel C) in HbS/FIIWT mice. Areas of necrosis are marked by dotted lines (A-B,D) and infiltrating leukocytes are marked by arrowheads (D) in HbS/FIIWT mice. In HbS/FIIlox/− mice, the areas of coagulative necrosis were minimal with comparatively fewer congested blood vessels seen. (F-H) CD31 staining of liver vascular endothelial cells showing vascular congestion in HbS/FIIWT mice (shown by arrows) along with prominent rounded endothelial cells (H). These changes were minimal in HbS/FIIlox/− mice. No differences were observed in the microvascular density (MVD) in liver sections from HbA/FIIWT and HbA/FIIlox/− mice. (F-G) Objective, ×40. (H) Objective, ×100. Images are representative of 9 mice per experimental group. (E) Alanine transaminase (ALT) activity in the serum of HbS/FIIWT, HbS/FIIlox/−, HbA/FIIWT, and HbS/FIIlox/− mice. Each symbol represents an individual animal and lines represent median values. Mice used as transplant recipients were of similar ages (8-10 weeks) with equal numbers of males and females distributed among the experimental groups. Fully chimeric sickle or normal mice were followed for a year after transplantation. Cohorts were analyzed by Mann-Whitney U test and statistical significance between FIIWT and FIIlox/− chimeras is indicated by asterisks: *P < .05. n.s., not significant.
Discussion
In this study, we show that FII plays a significant role in inflammation and multiorgan pathologies in mice with SCD. Consistent with prior work,50 we found that even in SCD mice, reducing the circulating FII to 10% of normal was associated with a relatively mild prolongation in the PT and reduced markers of FIIa activity, but was not associated with spontaneous bleeding. Moreover, these mild increases in PT are well below clinical PT targets for safe and effective anticoagulation. Yet, there was an impressive reduction in inflammation or associated reactive changes: there was significantly diminished (1) leukocytosis, specifically neutrophilia and monocytosis, (2) circulating IL-6, (3) inflammatory infiltrates in lung, liver, and kidneys, (4) vascular dysfunction (sVCAM-1), and (v) thrombocytosis. In addition, there was marked improvement in cardiac, renal, pulmonary, and hepatic histology and function: amelioration of RV hypertrophy and dilatation, albuminuria, and hyposthenuria. Of note, this reduction in inflammation and organ pathologies was not due to a change in the RBC parameters, suggesting no correlation of the inflammation with the underlying chronic hemolytic anemia or RBC turnover (reticulocytosis) with reduced circulating FII. These findings also suggest that reduction in FII is unlikely to limit hemolysis and free hemin-induced events, such as Toll-like receptor 4 (TLR-4) activation,68 although this has not been formally excluded. Rather, diminution of FII likely limited the vaso-occlusive manifestations of SCD and the secondary inflammation. An ultimate entirely objective indicator of the benefit of this intervention was the significantly improved survival of Berkeley sickle mice carrying low levels of FII.
A connection between sickle RBC, the hemostatic system, and SCD pathobiology has been long-speculated. Vaso-occlusions are the hallmark of SCD, and thrombotic changes secondary to sickling-induced vascular damage and sludging of blood flow are also innate to its pathophysiology. Consequently, the potential significance of procoagulant function in SCD has been recognized for decades. Prior studies of the anticoagulant vitamin K antagonist (VKA), warfarin, given during acute vaso-occlusive events (VOEs) suggested no effect on the duration of VOE in the short-term,69 and showed a modest reduction in VOE (from 1.3 VOE per year to 0.9 VOE per year) when given long-term. However, the authors acknowledged that the anticoagulation was poorly controlled and, therefore, the intervention had associated bleeding episodes in some patients.69 VKAs have the disadvantage of lowering all vitamin K–dependent factors, including the anticoagulant and anti-inflammatory protein C. Thus, based on these early findings, the benefits, of targeting the coagulation system, in general, remained uncertain, and any long-term benefits and liabilities of interventions at the level of a selected procoagulant have remained entirely unknown.
The present study unambiguously establishes, for the first time, that intervention at the level of a single key hemostatic factor, FII, can significantly ameliorate SCD organ pathologies and improve survival, at least in mice. Studies with a FII-specific ASO provides complementary proof-of-principle of the potential utility of pharmacologic intervention. A recent high-profile clinical trial using a FXI-specific ASO gapmer established their utility in safely attenuating postoperative venous thromboembolism.70 Although first-generation ASOs have been associated with proinflammatory effects,71 second-generation ASO gapmers are devoid of these side effects due to their advanced chemistries, high sequence specificity, and comprehensive screening strategies.72 Based on the present findings, it will be of keen interest to now also explore long-term benefits of interventions of this type or direct thrombin or FXa inhibitors in future studies of SCD. Although FII-specific ASO gapmers may have potential short-term utility in venous thromboembolism, their long-term use for the prevention of chronic organ damage in SCD may have compliance issues. In this regard, recent studies of SCD in mice intervening at the level of tissue factor (TF), the premiere initiator of the coagulation system, suggested suppressing TF activity reduced endothelial cell activation markers (sVCAM-1) and inflammatory markers (IL-6).42 Yet no such benefits were reported with regard to either of these metrics in mice treated with the oral FIIa inhibitor, dabigatran, in the short duration of 10 days.43 Our report demonstrates that intervention at the level of FII in sickle mice can mirror findings made previously with TF-blocking antibodies in reducing circulating sVCAM-1 and IL-6. However, the seeming discrepancy with prior dabigatran studies could simply reflect the distinct experimental observation periods (young adults studied for 10 days vs mice studied for 10-12 months) and the known progressive nature of sickle cell pathologies. It would not be surprising that some benefits of limiting FII become fully manifest over long time frames in sickle mice by reducing the cumulative tissue damage.
Recent studies have examined the components of the coagulation system in human subjects and in mouse models of SCD.44 Increased TF expression secondary to sickling and SCD-induced vascular endothelial/tissue damage is 1 likely driver of procoagulant activation. Recent evidence suggests that besides coagulation system activation from vascular endothelial/tissue damage, sickle RBC membrane damage, and PS exposure (plus PS-positive RBC microparticles) may be a significant driver of chronic thrombin generation and coagulation system activation, especially given their exceptional mass and turnover in SCD.34,73 Lowering FII in mice with SCD would be anticipated to limit the chronic procoagulant response to diminish both vaso-occlusion and inflammation. In addition to this, benefits of thrombin suppression could be gained at the level of both reduced platelet activation and reduced reactive changes leading to thrombocytosis.26 Although we did not examine platelet activation, our data suggest that part of the thrombocytosis in the HbS/FIIlox/− mice was reactive, secondary to the chronic inflammation that results from increased thrombin generation.
Although the precise mechanism(s) tying FII to SCD have not yet been formally established, at least 2 primary proteolytic targets of FIIa are strong candidates to support SCD-induced thromboinflammatory disease. First, fibrin(ogen), a known modifier of inflammatory diseases, may provide a local cue supporting leukocyte activation, especially macrophages, via the αMβ2 ligand, located on the γ-chain of fibrin(ogen).74 The availability of Fibγ390-396A mice carrying a mutant form of fibrinogen lacking the primary αMβ2-binding motif, but supporting clotting function, provide an attractive means for testing the concept that this thrombin target is coupled to SCD pathophysiology without imposing a hemorrhagic risk.75 The viability of mice lacking other thrombin substrates, such as the fibrin-stabilizing transglutaminase FXIII, provides an opportunity for more detailed mechanistic studies. If indeed dampening the proinflammatory effect of fibrin(ogen) offers benefit in SCD, the wholesale absence of clotting function is likely to be distinctly counterproductive, as the complete absence of fibrin(ogen) in an established, albeit far milder, model of SCD (transgenic SAD mice) resulted in increased mortality.76 A second, and not mutually exclusive, target of FIIa that may be mechanistically coupled to SCD outcome is PAR-1. Proteolytic activation of PAR-1 on endothelial cells results in a spectrum of functional changes that could be linked to SCD pathobiology, including changes in adhesive properties, the elaboration of hemostatic proteins, and changes in vascular permeability. Although short-term studies in PAR-1–deficient mice with SCD did not show significant benefits,43 longer-term studies may be needed to better define any PAR-1 and/or PAR-4 involvement.
Increased TF expression in monocytes and endothelium and monocyte-TF–bearing microparticles have been reported in patients with SCD13,25 and been associated with SCD pathologies including increased peak pulmonary artery pressures; however, the reports have been controversial, with some studies showing an association and others not.14,77-79 Studies on mouse models of SCD lend credence to the clinical associations while also illuminating causality. Here, we show that a genetically imposed chronic deficit in prothrombin, leading to a relatively modest increase in PT, is a major modifier of inflammation and organ pathology in SCD mice. A prothrombotic state is known to predispose to pulmonary microemboli and pulmonary vascular pruning in non-SCD–associated PHT.80 We show that this also occurs in SCD, where reduction in procoagulant function prevents development of PHT. In addition, we show a pleiotropic amelioration of multiorgan dysfunction. Of note, the remarkable improvement in glomerulopathy (reduction in albuminuria and FSGS), lung, and liver pathology implies that this is a primarily microvascular effect because these tissues are rich in capillaries. Reduced thrombin-mediated endothelial cell activation, as evidenced by plasma sVCAM-1 levels (and thereby reduced RBC and white blood cell [WBC] endothelial cell adhesion) and leukocytosis in HbS/FIIlox/− mice likely reduces microvascular blockade, as was observed in liver sections. Although Gavins et al have shown that direct inhibition of thrombin acutely via antithrombin III or hirudin reversed the accelerated experimentally induced thrombus formation in large vessels subjected to light/dye injury induced cerebral thrombosis in sickle mice,17 we show that chronic reduction in FIIa also limits microvascular thrombosis and endothelial dysfunction, impeding chronic end-organ damage. Its effect on stroke prevention, however, could not be elucidated, as the SCD mouse model is not a good model of unprovoked stroke. Overall, our studies establish that prothrombin diminution imparts substantial long-term benefit to multiple organs in SCD mice under normoxic day-to-day conditions, likely from reduced vascular occlusions, at least in the liver. It will be of added interest to explore whether short-term benefits are also gained in SCD mice when subjected to hypoxia4 or other secondary challenges (eg, tumor necrosis factor [TNF] α).81
Several human trials with anticoagulants45,82 and antiplatelet agents83-89 have been conducted, but have mostly been nonrandomized, nonplacebo controlled studies in small cohorts of patients. These studies have been limited by complex end points such as SCD-associated pain, which have thus far yielded mixed results. Nevertheless, these studies have shown that some of the anticoagulant and antiplatelet agents can be safely given to patients with SCD without inordinate risk of bleeding.45,82-89 Two placebo-controlled studies of acencoumarol and tinzaparin have been performed,90,91 of which the large randomized placebo-controlled trial by Qari et al has shown a significant effect of tinzaparin, a low-molecular-weight heparin, in reducing VOE.91 However, except for 1 anecdotal report on healing of leg ulcers published nearly 60 years ago, none of the clinical trials have attempted to examine the effect of reduced procoagulant function on end-organ damage in SCD, which worsens asymptomatically over long periods and only becomes clinically apparent when overt organ failure ensues. We show, for the first time, that long-term intervention at the level of a single hemostatic factor, prothrombin, is sufficient for a system-wide anti-inflammatory effect, reduced endothelial cell activation, and amelioration of organ pathology and dysfunction and significantly improved survival in SCD mice, without hemorrhagic events. Our studies open a new paradigm of targeting excessive thrombin generation or activity as a novel means to limit multiorgan damage in SCD. The availability of new orally bioavailable FIIa, FXa, and PAR-1 inhibitors designed to suppress selected procoagulants suggest that translation of these results may be possible in the near future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Betsy Dipasquale for help with immunohistochemistry, Christopher Woods for help with illustrations, Adam Lane for statistical analysis, and the Cincinnati Children’s Hospital Medical Center Pathology Core for histopathology.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute R01 HL112603 (multiple principal investigators [MPI]) grant (J.L.D. and P.M.).
Authorship
Contribution: P.I.A., A.L., M.A.S., and E.S.M. performed the experiments and plotted the data; S.K.S. scored and interpreted tissue histology; J.W. performed and analyzed CMR; T.R. performed immunohistochemistry; J.L.D., P.M., P.I.A., B.P.M., and E.S.M. designed the experiments and interpreted the overall experimental data; P.I.A., P.M., E.S.M., and J.L.D. wrote the manuscript; and all authors provided their comments on the manuscript.
Conflict-of-interest disclosure: B.P.M. is an employee of Isis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Punam Malik, Cincinnati Children’s Hospital Medical Center, Mail Location 7013, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: punam.malik@cchmc.org; and Jay Degen, Cincinnati Children’s Hospital Medical Center, Mail Location 7013, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: jay.degen@gmail.com.
References
Author notes
P.I.A. and E.S.M. contributed equally.
The two senior authors J.L.D. and P.M. contributed equally.